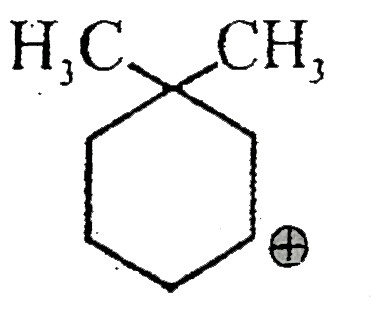
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
ALLEN-TEST PAPERS-PAPER 1
- Out of following which will show hyperconjugation:-
Text Solution
|
- Identify the major product?
Text Solution
|
- Rearrangement is not possible in :-
Text Solution
|
- Assertion: If d(x^(2)-y^(2)) and p(y) orbitals come close together al...
Text Solution
|
- Assertion:- CH(3)Cl has more dipole moment than CH(3)F Reason:- The ...
Text Solution
|
- Assertion: - Metaphosphoric acid is highly unstable in monomer form an...
Text Solution
|
- Explain, why does boron not form B^(3+) ions.
Text Solution
|
- In which process Ca(OH)(2) is used to produce NH(3)?
Text Solution
|
- Softening of hard water is done using sodium aluminium silicate (zeoli...
Text Solution
|
- Aqueous ammonia is used as a precipitating reagent for Al^(3+) ions as...
Text Solution
|
- Assertion:- The reaction quotient Q, has the same presentation form as...
Text Solution
|
- Assertion:- H(3)PO(4) is a dibasic compound. Reason:- The two H-ato...
Text Solution
|
- Assertion:- If some PCl(3)(g) containing labelled (radio active) phosp...
Text Solution
|
- Assertion (A): Addtion of NH(4)OH to an aqueous solution of BaCl(2) in...
Text Solution
|
- When the system does not exchange heat with the surroundings, the proc...
Text Solution
|
- Assertion:- Reversible process and a cyclic process are the same typ...
Text Solution
|
- Assertion:- 2nd member of ethyl ketone forms two oximes on reacting wi...
Text Solution
|
- Assertion:- Boiling point of cis-2-butene is more than trans-2-butene....
Text Solution
|
- Assertion:- Acrylic acid is unsaturated compound. Reason:- One doub...
Text Solution
|
- Assertion:- All compounds having C=C bond exhibit geometrical isomeris...
Text Solution
|