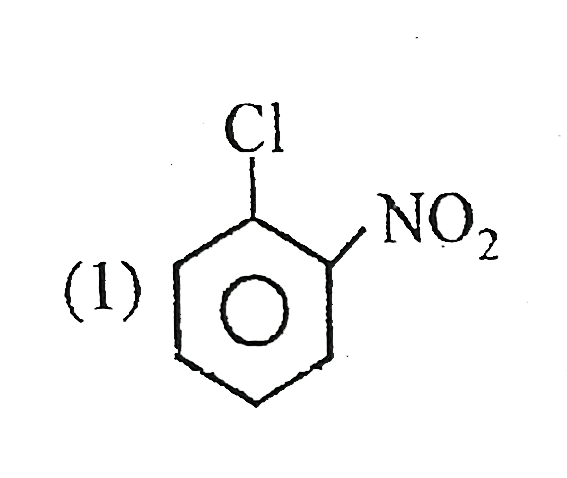
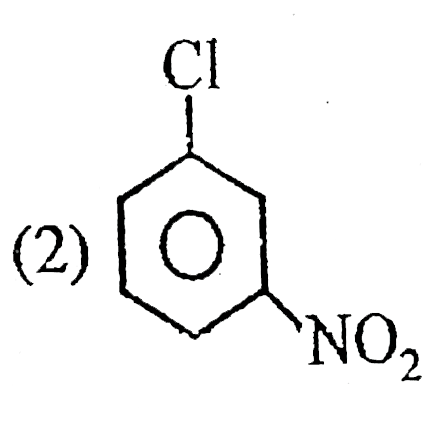
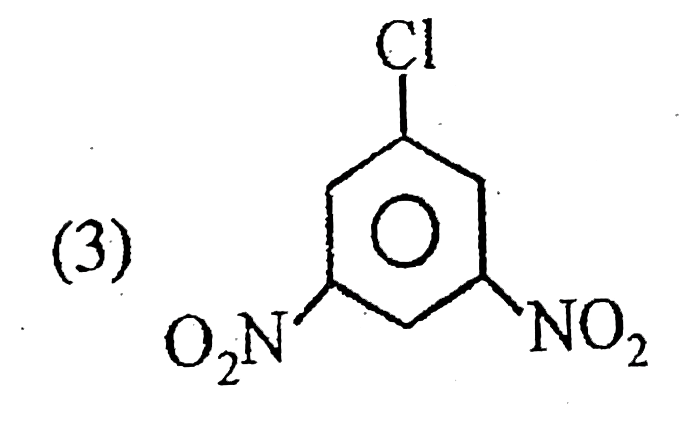
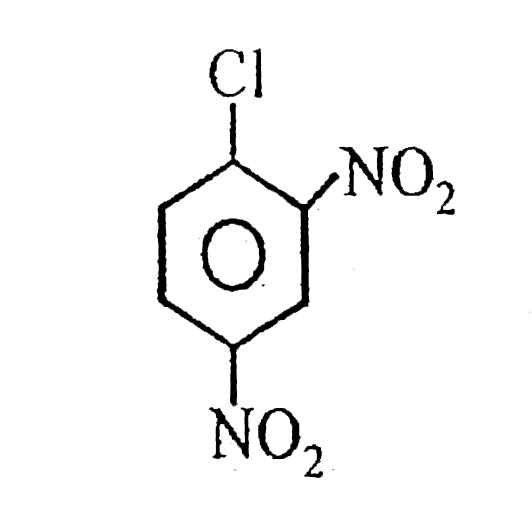
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPERS-PAPER 2
- What could be the product for following reaction?
Text Solution
|
- The major product of following reaction is :-
Text Solution
|
- Which one of the following compounds will be most readily hydrolyse...
Text Solution
|
- In the given reaction :- The final product (Y) is :
Text Solution
|
- What could be the product for the following reaction ?
Text Solution
|
- What could be the product for the following reaction ?
Text Solution
|
- Among the following reaction, which from salicylic acid.
Text Solution
|
- Among the following polymer, identify compolymer ?
Text Solution
|
- Identify structure of Adenine :-
Text Solution
|
- Which of the following is not a natural polymer ?
Text Solution
|
- Mathc the column (I) and column (II) :-
Text Solution
|
- overset((i)CH(3)CO(3)H)rarr product is:-
Text Solution
|
- Complete the following reaction
Text Solution
|
- In the given reaction (A) is :-
Text Solution
|
- Assertion (A): The colour of old lead painting can be restored by wash...
Text Solution
|
- Assertion:- K(3)[Fe(CN)(6)] is an example of low spin complex. Reaso...
Text Solution
|
- Assertion:- Boric acid cannot be titrated with NaOH solution. Reason...
Text Solution
|
- Assertion : The heavier p-block element do not form strong pi bonds. ...
Text Solution
|
- Assertion : The heavier p-block element do not form strong pi bonds. ...
Text Solution
|
- Assertion (A) SF(6) cannot be hydrolysed but SF(4) can be. Reason...
Text Solution
|