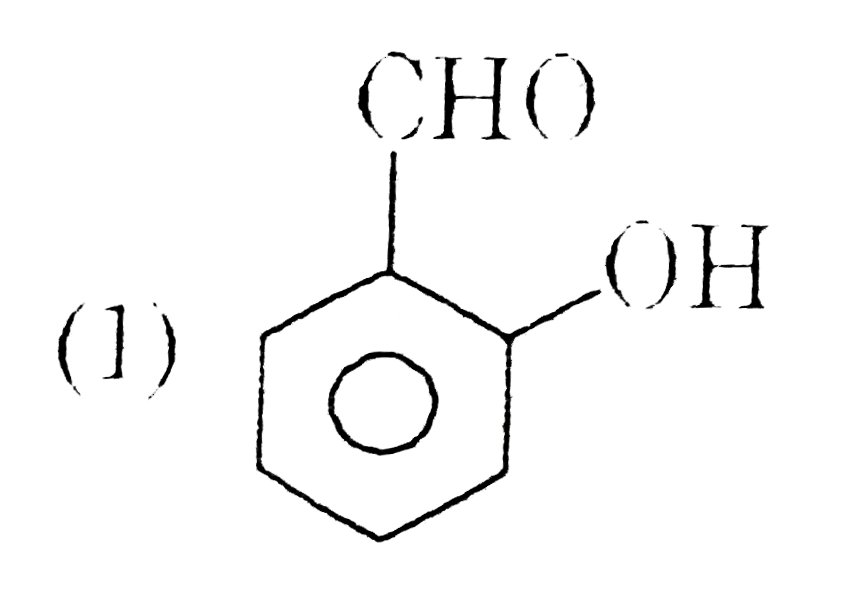
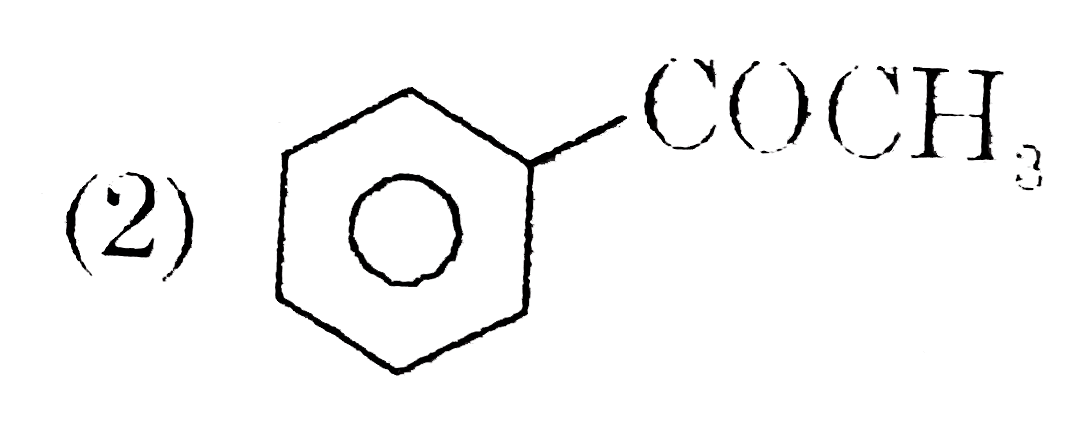
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPERS-part-2 Chemistry
- A and B respectively are
Text Solution
|
- C(2)H(5)Cl+Naunderset(-NaCI)overset("dry ether")(rarr)A. How many isom...
Text Solution
|
- Which of the following will undergo self aldol reaction on heating wit...
Text Solution
|
- Calculate the percentage of SN^(-1) product, if (R )-2-Chloro butane o...
Text Solution
|
- CH(3)-CHO & C(6)H(5)-CO-CH(3) can reacts similarly with
Text Solution
|
- overset(HI)underset("low temp")rarr product are
Text Solution
|
- The major product of the following reaction will be
Text Solution
|
- The suitable reagent to convert CH(3)-CH=CH-CHO into CH(3)-CH(2)-CH(2...
Text Solution
|
- In the reaction sequence CH(2)=CH(2)underset(C Cl(4))overset(HBr)(ra...
Text Solution
|
- Determine the potential of the following cell: Pt|H2(g,0.1 "bar")|H^...
Text Solution
|
- The Boyle temperature of three gases are given in the table : {:("Ga...
Text Solution
|
- If 250 mL of N(2) over water at 30^(@)C and a total pressure of 740 t...
Text Solution
|
- If the density of a mixture of O(2)&N(2) at N.T.P. is 1.258 g//l. The...
Text Solution
|
- An element X having vapour density equal to 40 in gaseous state underg...
Text Solution
|
- It was found that in 30 unit cells of ZnS, four atoms are of trivalent...
Text Solution
|
- A 45 ml mixture of hydrogen and oxygen is sparked to form liquid water...
Text Solution
|
- H(2)O(2)+2KIoverset(40% "yield")rarrI(2)+2KOH H(2)O(2)+2KMnO(4)+3H(2...
Text Solution
|
- In the reaction, I(2)+2S(2)O(3)^(2-) rarr 2I^(-)+S(4)O(6)^(2-).
Text Solution
|
- conductometric titration curve of a equimolar mixture of a HCl and HCN...
Text Solution
|
- 100 ml 0.04 M HA (K(a)=10^(-5)) and 100 mI 0.8 M HB (K(a) = 10^(-6)) ...
Text Solution
|