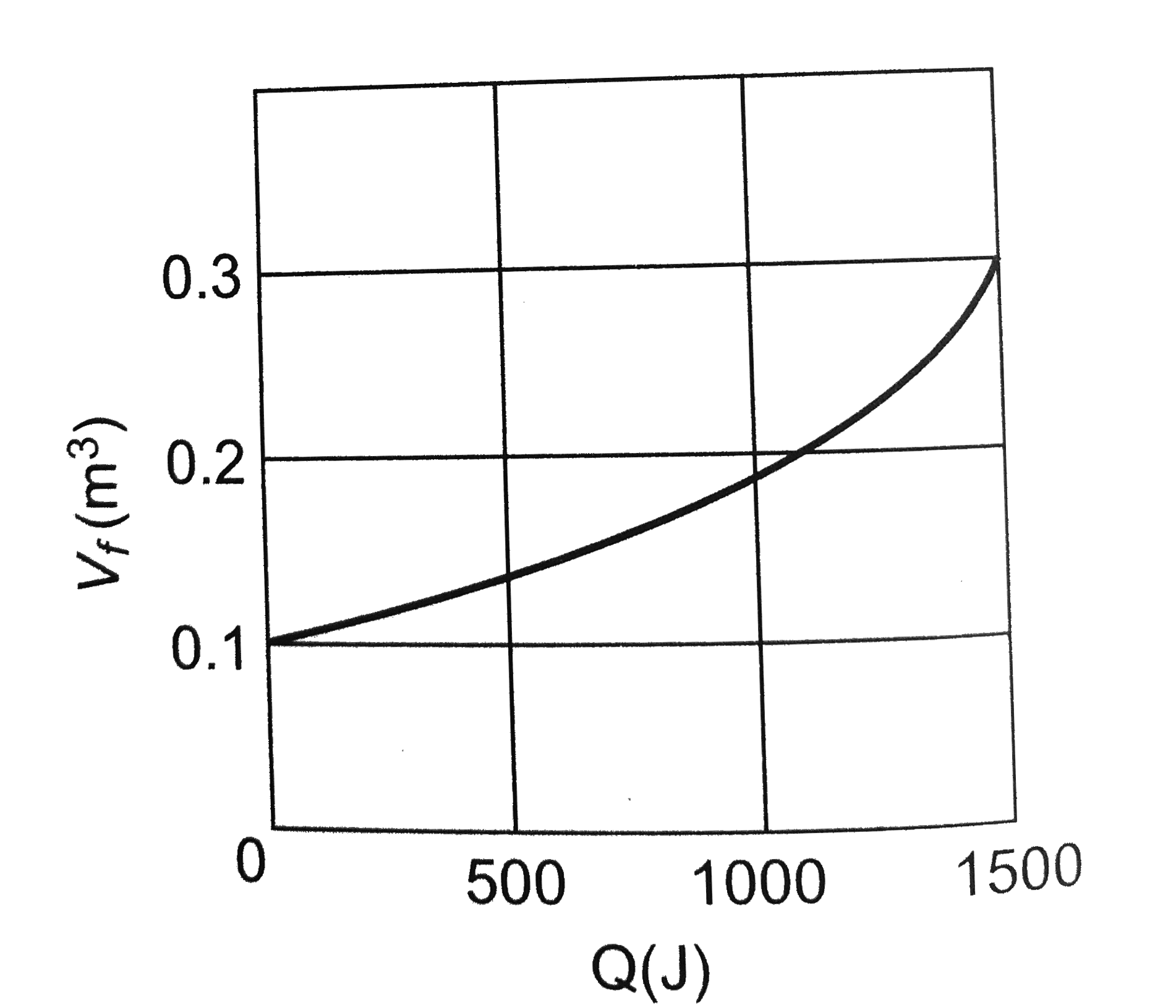
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-GEOMETRICAL OPTICS-SOME WORKED OUT EXAMPLES
- Figure demonstrates a polytropic process (i.e.PV^(n) = constant ) for ...
Text Solution
|
- A vessel contains 14 g (7 moles ) of hydrogen and 96 g (3moles) of oxy...
Text Solution
|
- Suppose 0.5 moles of an ideal gas undergoes an isothermal expansion as...
Text Solution
|
- The respective speeds of five molecules are 2,1.5,1.6,1.6 and 1.2 km/s...
Text Solution
|
- When water is boiled at 2 atm pressure the latent heat of vaporization...
Text Solution
|
- Given T-p curve for three processes. Work done in process 1, 2 and 3 (...
Text Solution
|
- Figure shows the variations of the internal energy U With density rho ...
Text Solution
|
- One mole of an ideal gas undergoes a process whose molar heat capacity...
Text Solution
|
- An irregular rod of same uniform material as shown in figure is condu...
Text Solution
|
- A gas undergoes an adiabatic process in which pressure becomes ((8)/(3...
Text Solution
|
- Following graphs shows the variation in the intensity of heat radiatio...
Text Solution
|
- The temperature drop through a two layer furnace wall is 900^(@)C. Eac...
Text Solution
|
- 5g of steam at 100^(@)C is mixed with 10g of ice at 0^(@)C. Choose cor...
Text Solution
|
- Water contained in a jar at room temperature (20^(@)C) is intended to ...
Text Solution
|
- n moles of an ideal triatomic linear gas undergoes a process in which ...
Text Solution
|
- An ideal gas expands in such a way that PV^2 = constant throughout the...
Text Solution
|
- Which of the following processes must violate the first law of thermod...
Text Solution
|
- For an ideal gas of fixed amount, the initial pressure and volume are...
Text Solution
|
- In Newton's law of cooling (d theta)/(dt)=-k(theta-theta(0)) the const...
Text Solution
|
- The graph below shows V-p curve for three processes. Choose the correc...
Text Solution
|