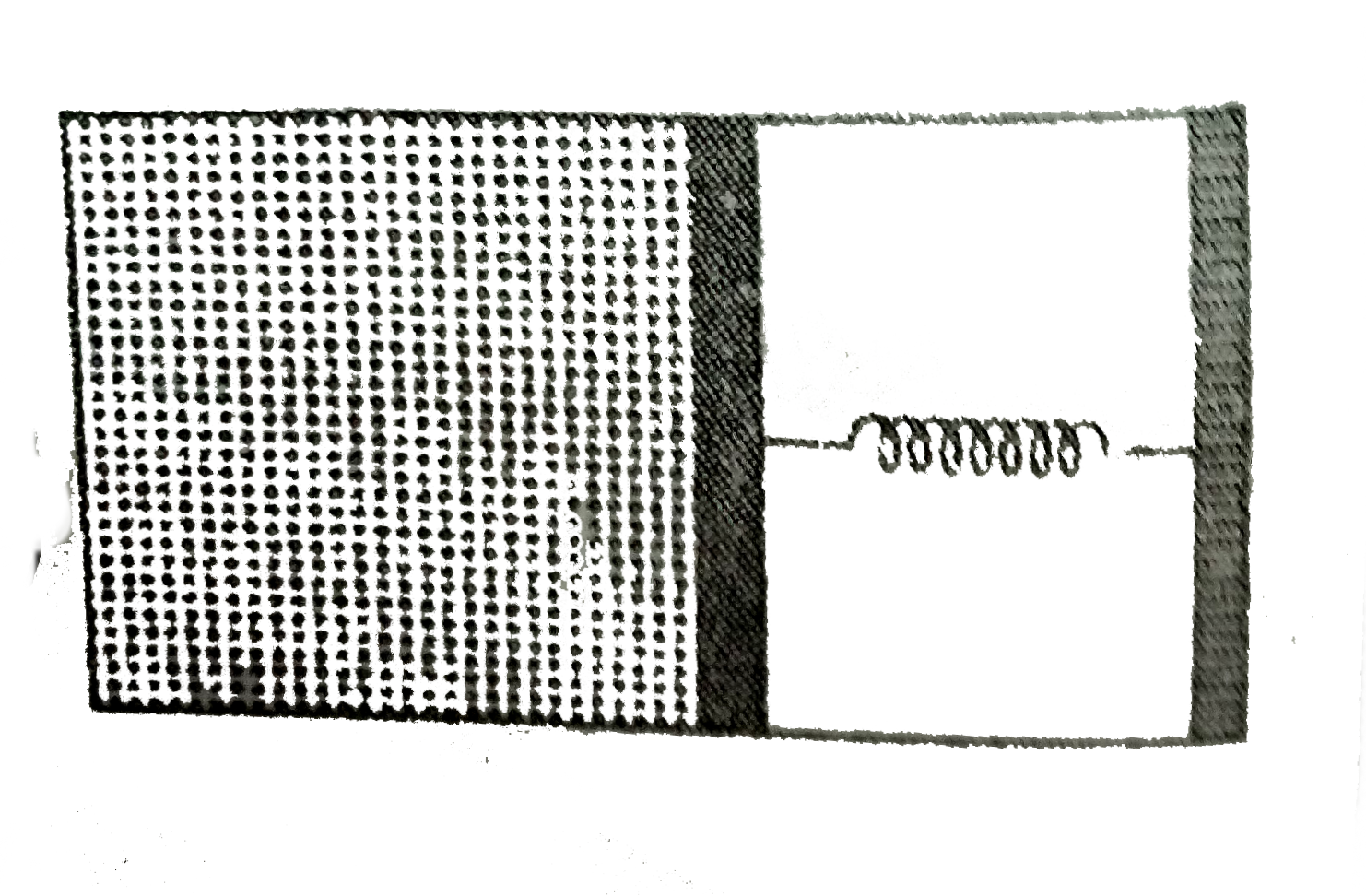
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GEOMETRICAL OPTICS
ALLEN|Exercise EXERCISE - 05 (B) (MATCH THE COLUMN)|3 VideosGEOMETRICAL OPTICS
ALLEN|Exercise EXERCISE - 05 (B) (ASSERTION & REASON )|9 VideosGEOMETRICAL OPTICS
ALLEN|Exercise EXERCISE - 05 (B)|58 VideosCURRENT ELECTRICITY
ALLEN|Exercise EX.II|66 VideosGRAVITATION
ALLEN|Exercise EXERCISE 4|9 Videos
Similar Questions
Explore conceptually related problems
ALLEN-GEOMETRICAL OPTICS-EXERCISE - 05 (B) (MCQ)
- Let vecV, V("m s") and Vp respectively, denote the mean speed, root ...
Text Solution
|
- During the melting of a slab of ice at 273 K a atmospheric pressure
Text Solution
|
- A bimetallic strip is formed out of two identical strips one of copper...
Text Solution
|
- Cv and Cp denotes the molar specific heat capacities of a gas at con...
Text Solution
|
- Figure shows the P-V plot of an ideal gas taken through a cycle ABCDA....
Text Solution
|
- The figure below shows the variation of specific heat capacity (C) of ...
Text Solution
|
- A container of fixed volume has a mixture of one mole of hydrogen and ...
Text Solution
|
- In plotting stress versus strain curves for two materials P and Q, a s...
Text Solution
|
- An ideal monatomic gas is confined in a horizontal cylinder by a sprin...
Text Solution
|