Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SIMPLE HARMONIC MOTION
ALLEN|Exercise Exercise-05(B)|46 VideosSIMPLE HARMONIC MOTION
ALLEN|Exercise MCQ s|8 VideosSIMPLE HARMONIC MOTION
ALLEN|Exercise Exercise - 58|1 VideosRACE
ALLEN|Exercise Basic Maths (Wave Motion & Dopplers Effect) (Stationary waves & doppler effect, beats)|24 VideosTEST PAPER
ALLEN|Exercise PHYSICS|4 Videos
Similar Questions
Explore conceptually related problems
ALLEN-SIMPLE HARMONIC MOTION-Exercise - 04[A]
- What is the effect on the maximum kinetic energy and the numver of pho...
Text Solution
|
- A small 10 W source of wavelength 99 nm is held at a distance 0.1 m fr...
Text Solution
|
- An electron of mass m and magnitude of change |e| initially at rest ge...
Text Solution
|
- Let us assume that the de-Broglie wave asssociated with an electron fr...
Text Solution
|
- An electron, in a hydrogen like atom , is in excited state. It has a t...
Text Solution
|
- An electron in the ground state of hydrogen atom is removing in unanti...
Text Solution
|
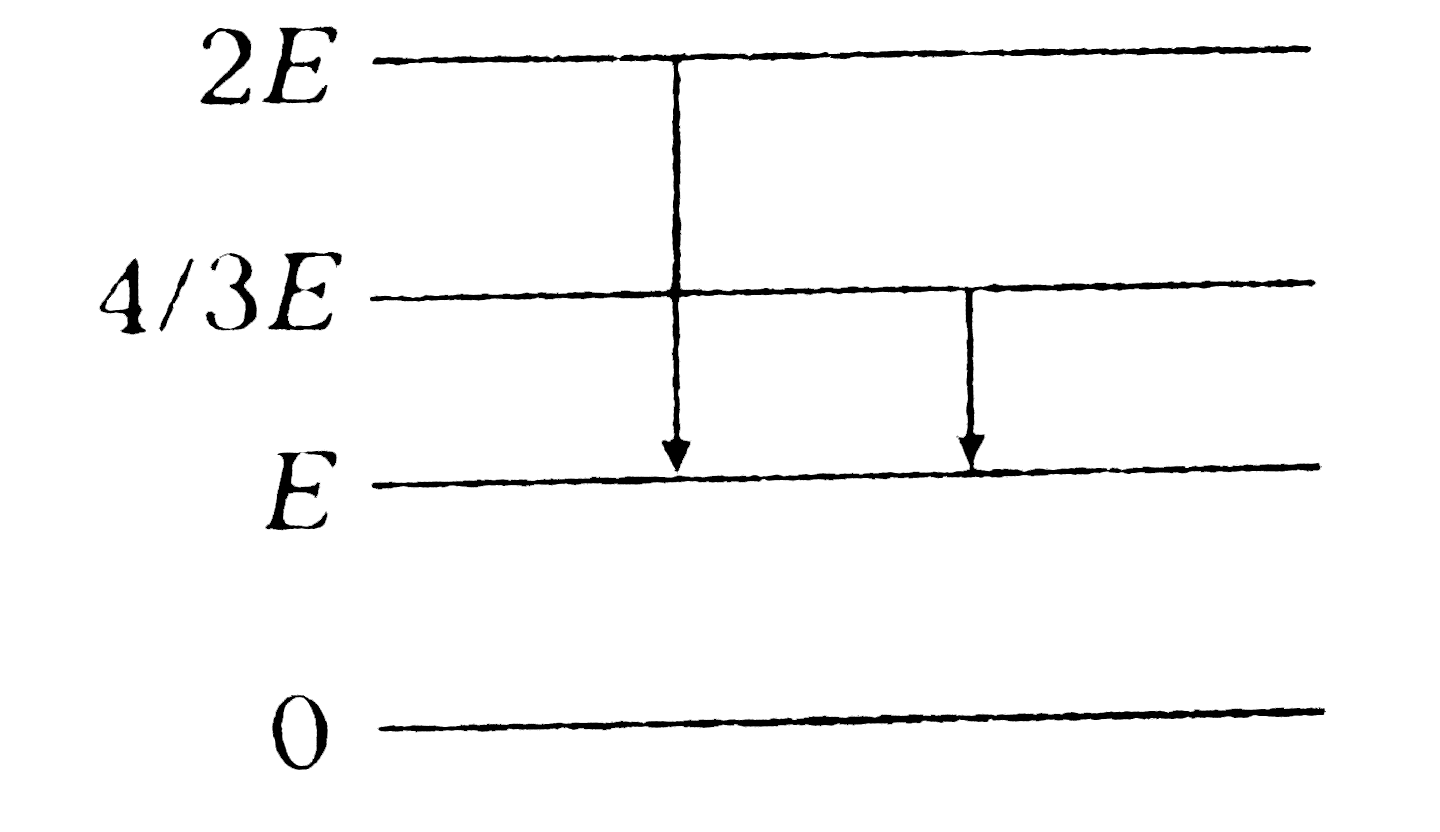
- The energy levels of a certain atom are represented in adjoining figur...
Text Solution
|
- A hydrogen like atom (atomic number Z) is in a higher excited state o...
Text Solution
|
- Find the ratio of series limit wavelength of Balmer series to waveleng...
Text Solution
|
- An electron joins a helium nucleus to form He^(+). Find the wavelength...
Text Solution
|
- An X-ray tube, operated at a potential difference of 40 kV, produce he...
Text Solution
|
- The Ka X-ray of molybdenum has wavelength 71 pm. If the energy of a mo...
Text Solution
|
- The positron is a fundamental particle with the same mass as that of t...
Text Solution
|
- The binding energies per nucleon for deuteron (1H^2) and helium (2He^4...
Text Solution
|
- If there times of lambda(min) of continuous X-ray spectrum of target m...
Text Solution
|
- Decay constant of two radioactive samples is lambda and 3lambda respec...
Text Solution
|
- The half lives of radioactive elements X and Y are 3 mintue and 27 min...
Text Solution
|
- 36% amount of a radioactive sample disintegrates in t time. Calculate ...
Text Solution
|
- By using the following atomic masses : .(92)^(238)U = 238.05079u. .(2)...
Text Solution
|
- A radioactive sample decays with an average life of 20 ms. A capacitor...
Text Solution
|