Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ACIDIC STRENGTH & BASIC STRENGTH
ALLEN|Exercise Exercise III|14 VideosACIDIC STRENGTH & BASIC STRENGTH
ALLEN|Exercise Exercise IV|30 VideosACIDIC STRENGTH & BASIC STRENGTH
ALLEN|Exercise Exercise V|16 VideosCONCENTRATION TERMS
ALLEN|Exercise Exercise Jee-Advance|3 VideosAIIMS 2019
ALLEN|Exercise CHEMISTRY|40 Videos
Similar Questions
Explore conceptually related problems
ALLEN-ACIDIC STRENGTH & BASIC STRENGTH-Exercise II
- Write decreasing order of basic strength of following:
Text Solution
|
- Arrange the following compounds in decreasing order of their basicity?...
Text Solution
|
- Correct decreasing order of basic strength: Of following compound...
Text Solution
|
- The decreasing order of basic characters of the following is : (I) A...
Text Solution
|
- The decreasing order of basic characters of the following is : (I) A...
Text Solution
|
- Which one of the following is least basic in character?
Text Solution
|
- In each of the following pair of compounds,w hich is more basic in aqu...
Text Solution
|
- Choose the member of each of the following pairs of compounds that is ...
Text Solution
|
- Explain which compound is the wekar base. .
Text Solution
|
- Arrange the basic strength of the following compounds: .
Text Solution
|
- Arrange the following compounds in order of increaing basicity. (a)....
Text Solution
|
- Which of the following si most basic:
Text Solution
|
- Basicity order of N in following compound is: .
Text Solution
|
- The conjugate base of serotonin (used as tranquilisers) is given as fo...
Text Solution
|
- The structure of saccharin is given as follows: How many followin...
Text Solution
|
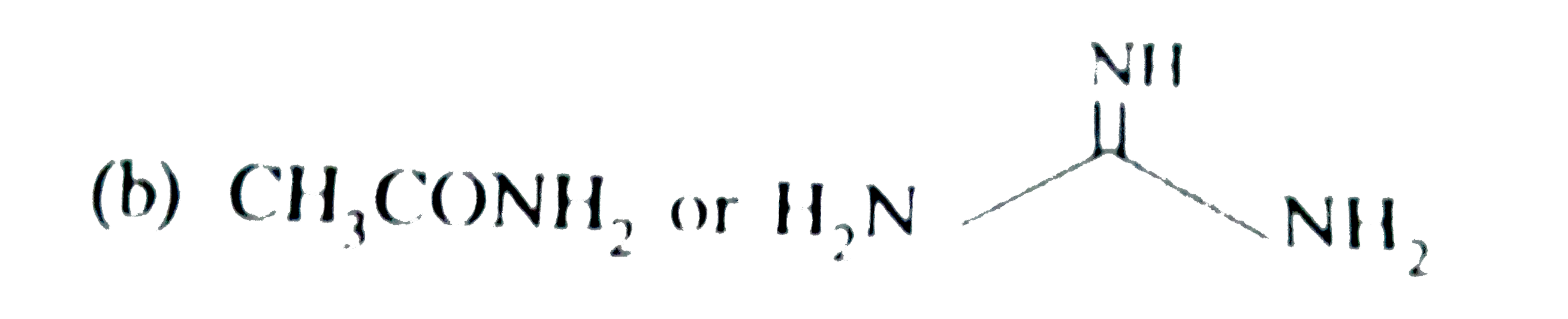 ltBrgt (c). `CH_(3)CH_(2)CH_(2)NH_(2)` or `CH_(3)CN`
ltBrgt (c). `CH_(3)CH_(2)CH_(2)NH_(2)` or `CH_(3)CN`