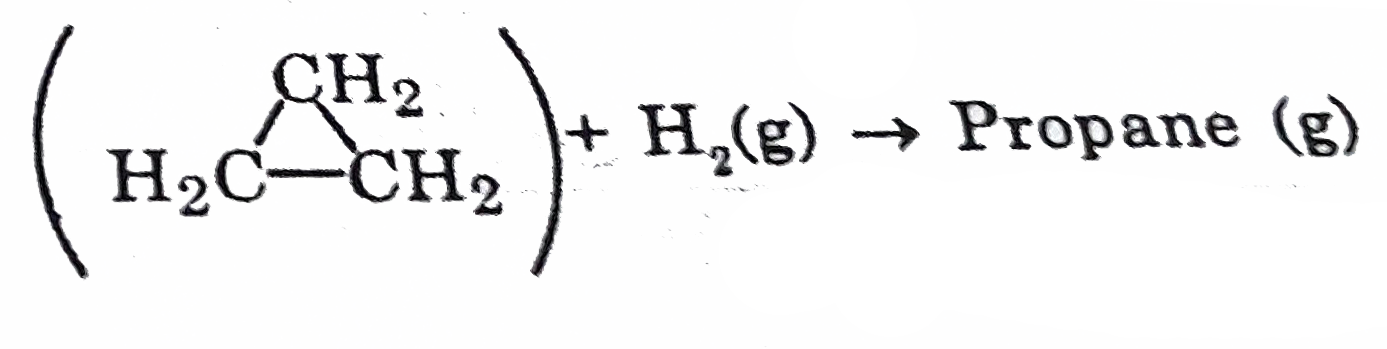A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPERS-CHEMISTRY
- Calculate solubility (in moles/litre) of a saturated aqueous solution ...
Text Solution
|
- Given 0.158 mol of acidified Aluminium dichromate will oxidise how man...
Text Solution
|
- Calculate DeltaH for the reaction - Cyclopropane (g) Give...
Text Solution
|
- Which of the following metallurgical process for the extraction of ind...
Text Solution
|
- Which of the following compound gives yellowish white turbidity with d...
Text Solution
|
- Which of following shows highest rate of decarboxylation in presence o...
Text Solution
|
- Aoverset("KCN")toBoverset(H(3)O+)underset(NH(3)//Delta)toCoverset(Br(2...
Text Solution
|
- Choose the incorrect statement (s )-
Text Solution
|
- Three different solutions of oxidising agents KMnO(4),K(2)Cr(2)O(7) " ...
Text Solution
|
- Which of the following reactions (s) is/are correctly represented to p...
Text Solution
|
- The CORRECT statement (s) is/are:
Text Solution
|
- Which of following gives positive carbylamine test ?
Text Solution
|
- Correct set of reaction which produce carbanion intermediate is/are
Text Solution
|
- Acylation followed by clemenson reduction is more suitable to get alky...
Text Solution
|
- Arrhenius studies the effect of temperature on the rate of a reaction...
Text Solution
|
- Arrhenius studies the effect of temperature on the rate of a reaction...
Text Solution
|
- Which of the following sulphide(s) does/do not liberate H(2)S on warmi...
Text Solution
|
- Which of the following oxide is basic?
Text Solution
|
- Select the INCORRECT statement .
Text Solution
|
- Select the INCORRECT statement regarding hydrolysis of SF(4)
Text Solution
|