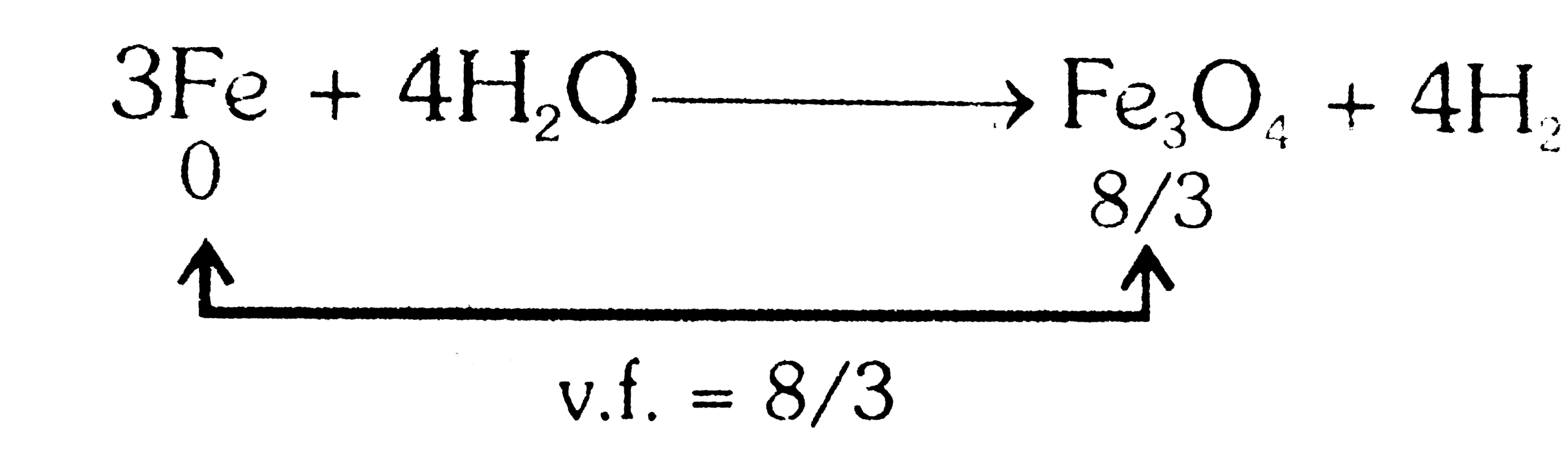A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-REDOX REACTIONS-EXERICSE - 2
- The oxidation number of arsenic atom in H(3)AsO(4) is :-
Text Solution
|
- In substance Mg(HXO(3)), the oxidation number of X is :-
Text Solution
|
- In the following change 3Fe+4H2OrarrFe3O4 +4H2 If the atomic weight of...
Text Solution
|
- In the course of a chemical reaction an oxidant -
Text Solution
|
- Which statement is wrong?
Text Solution
|
- Identify the correct statement with respect to the following reaction,...
Text Solution
|
- Which of the following reactions involves neither oxidation nor reduct...
Text Solution
|
- In the reaction :- C+4HNO(3)rarrCO(2)+2H(2)O+4NO(2) HNO(3) acts as...
Text Solution
|
- In the following reaction the value of 'X' is H(2)O+SO(3)^(2-)rarr S...
Text Solution
|
- The oxidation number of P in KH(2)PO(3) is ..........
Text Solution
|
- The oxidation number of iron in potassium ferricyanide [K(3)Fe(CN)(6)]...
Text Solution
|
- The oxidation number of phosphorus in PH(4)^(+),PO(2)^(3-),PO(4)^(3-) ...
Text Solution
|
- Which of the following compounds are arranged in increasing oxidation ...
Text Solution
|
- Iodine shows the highest oxidation state in the compound :-
Text Solution
|
- The number of electrons required to balance the following equation are...
Text Solution
|
- Cr(2)O(7)^(-2)+I^(-)+H^(+)rarrCr^(+3)+I(2)+H(2)O The equivalent weig...
Text Solution
|
- The sum of the oxidation states of all the carbon atoms present in the...
Text Solution
|
- In the reaction 8Al+3Fe(3)O(4)rarr 4Al(2)O(3)+9Fe the number of el...
Text Solution
|
- In which of the following reactions, hydrogen is acting as an oxidisin...
Text Solution
|
- Oxidation number of silver in silver amalgam is
Text Solution
|