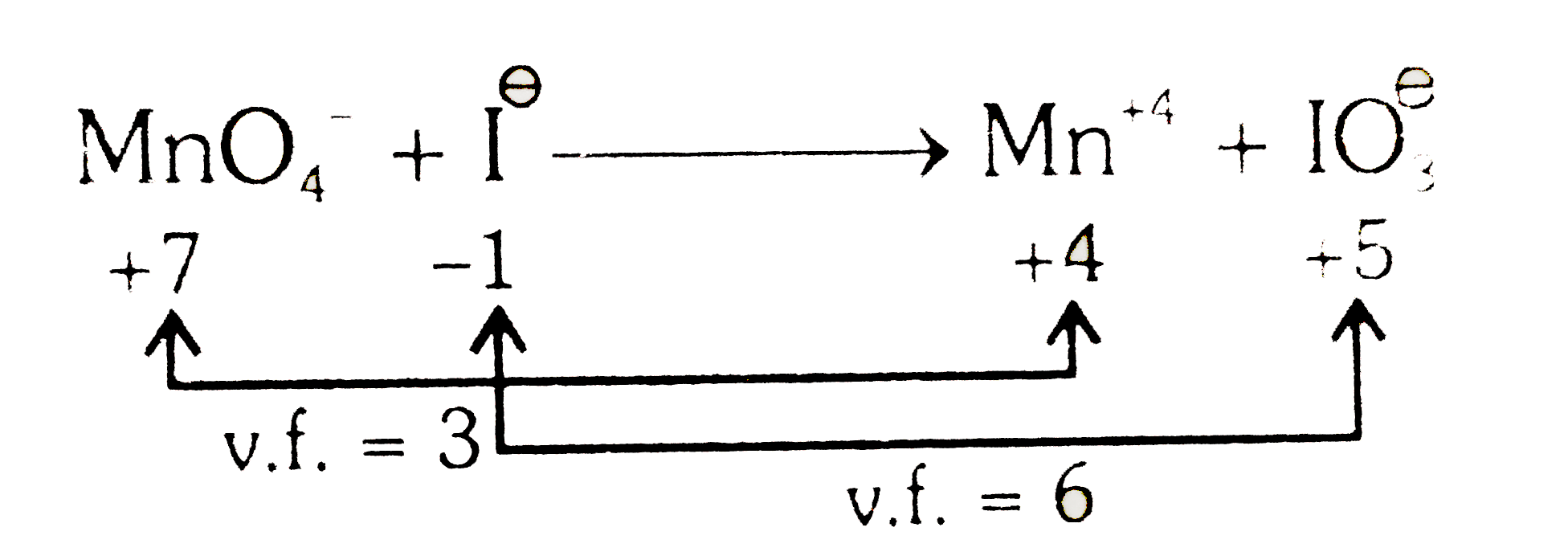A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-REDOX REACTIONS-EXERICSE - 3
- Zn+H(2)SO(4)rarrZnSO(4)+H(2) Zn undergoes -
Text Solution
|
- The oxidation state of Cr in Cr(2)O(7)^(2-) is
Text Solution
|
- The number of moles of KMnO(4) reduced by 1 "mol of" KI in alkaline me...
Text Solution
|
- The correct order of acidic strength is
Text Solution
|
- Number of moles of MnO(4)^(-) required to oxidise one mole of ferrous ...
Text Solution
|
- Oxidation numbers of P in PO(4)^(3-), of S in SO(4)^(2-) and that of C...
Text Solution
|
- What will be the oxidation number of Fe in K(3)[Fe(CN)(6)] :-
Text Solution
|
- In the reaction 2FeCl(3)+H(2)Srarr2FeCl(2)+2HCl+S
Text Solution
|
- For the redix reaction, MnO(4)^(-)+C(2)O(4)^(2-) +H^(+) to Mn^(2+)+C...
Text Solution
|
- Which of the following act both as oxidant & reductant :-
Text Solution
|
- Which of the following reaction is spontaneous oxidation - reduction r...
Text Solution
|
- For reaction : Cr(2)O(7)^(-2)+14H^(+)rarr2Cr^(+3)+7H(2)O, How many e...
Text Solution
|
- Oxidising agents have high :-
Text Solution
|
- The ration of coefficients of HNO3,Fe(NO3) and NH4NO3 in the following...
Text Solution
|
- What is the oxidation state of chlorine in perchloric acid.
Text Solution
|
- Correct order of oxidising strength is :-
Text Solution
|
- When S(2)O(8)^(2-) oxidise Fe^(2+) then product formed is :-
Text Solution
|
- The oxidation state of sulphur in H(2)SO(5) and chromium in K(2)Cr(2)O...
Text Solution
|
- How much amount of CuSO(4).5H(2)O required for liberation of 2.55g I(...
Text Solution
|
- When Cl(2) gas reacts with hote and concentrated sodium hydroxide solu...
Text Solution
|