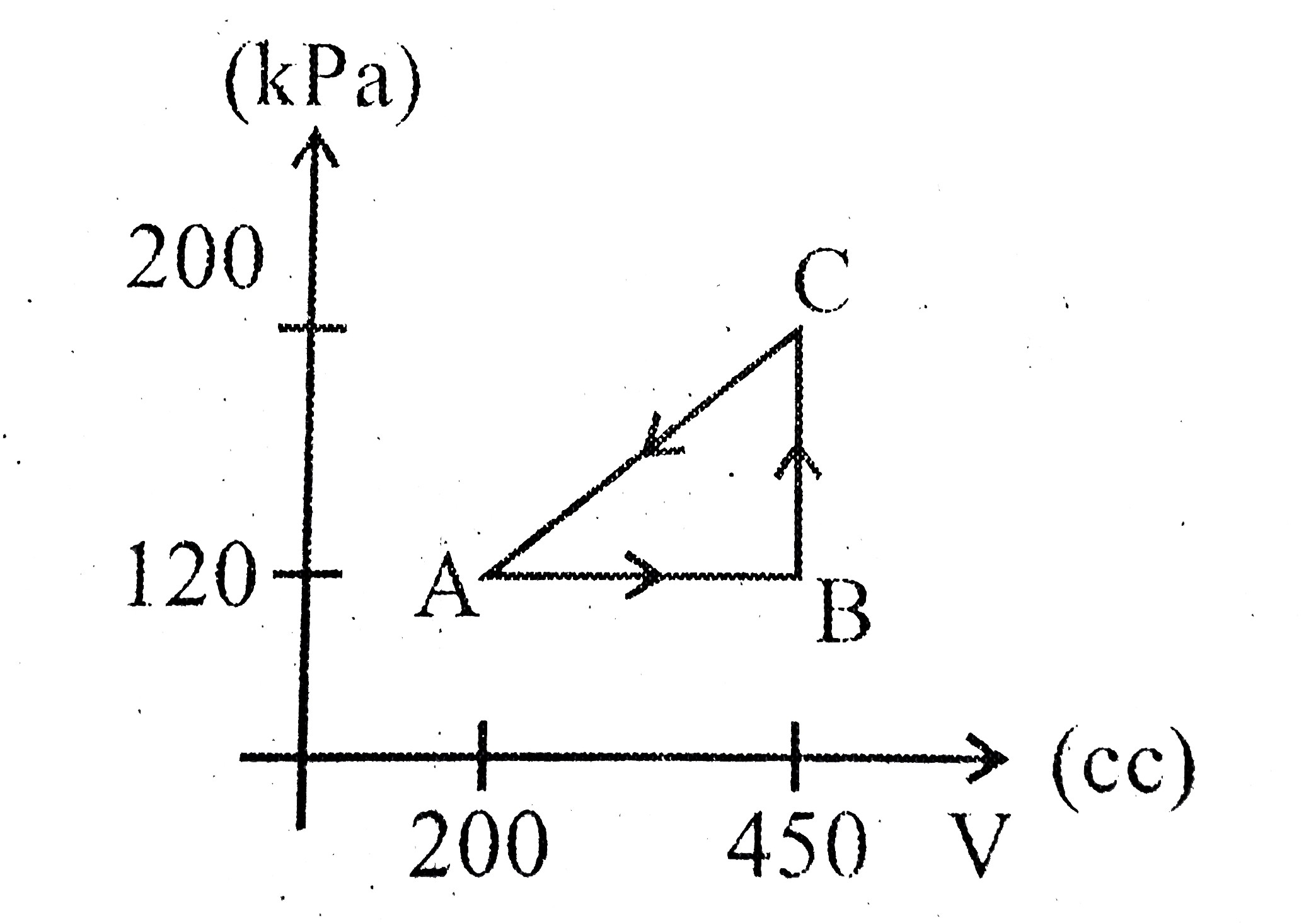
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPER 2-PHYSICS
- Thirteen resistors each of resistance R connected in the circuit as sh...
Text Solution
|
- The emf of the battery shown in figure is
Text Solution
|
- A piece of copper and another of germanium are cooled from room temper...
Text Solution
|
- The electron in hydrogen atom moves with a speed of 2.2xx10^(6)m//s in...
Text Solution
|
- The current in a wire varies with time according to the equation I = ...
Text Solution
|
- Calculate the resistance of a hollow cylindrical pipe of inner and out...
Text Solution
|
- The resistance of each side of an equilateral triangle is 6 ohm. Calcu...
Text Solution
|
- Find the effective resistance between points A and B for the network s...
Text Solution
|
- In the given figure when the key is open the ammeter reading is 5 amp....
Text Solution
|
- Four molecules of gas have speeds 1,2,3 and 4 km//s.The value of the r...
Text Solution
|
- At what temperature RMS velocity of O2 molecule will be one third of H...
Text Solution
|
- Figure shows graphs of pressure vs density for an ideal gas at two tem...
Text Solution
|
- During an experiment, an ideal gas is found to obey an additional law ...
Text Solution
|
- Q cal of heat is required to raise the temperature of 1 mole of a mona...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure the fraction...
Text Solution
|
- Internal energy of an ideal gas depends upon a) Temperature only b) vo...
Text Solution
|
- Calculate the work done by the gas in the state diagram shown :
Text Solution
|
- A = x + y and B = xy x = (20 pm1) cm , y = (10 pm 1) cm Then whic...
Text Solution
|
- Four Screw guages are given (A) Pitch = 1 mm, no. of division circu...
Text Solution
|
- Which of the following is fundamental vector quantity.
Text Solution
|