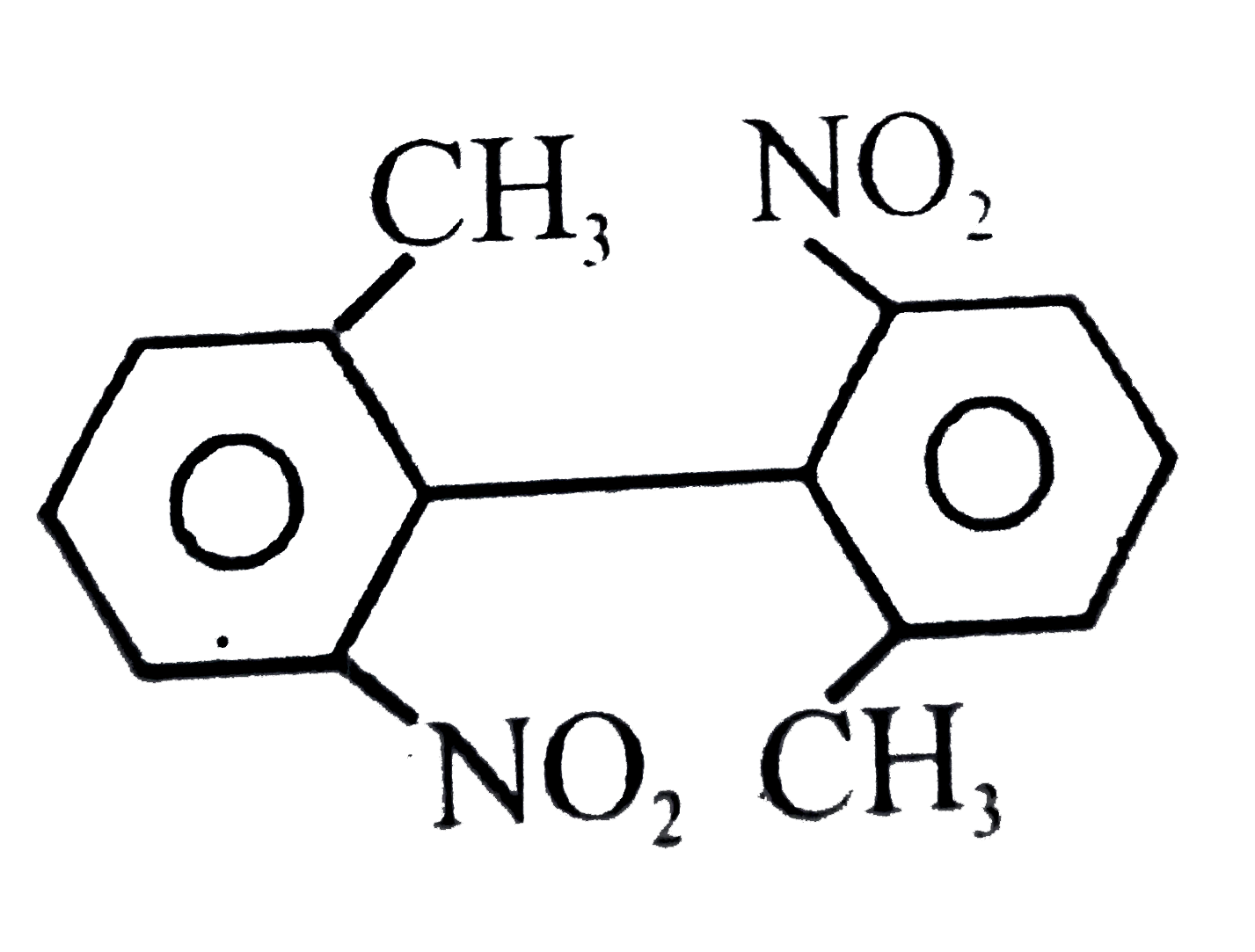
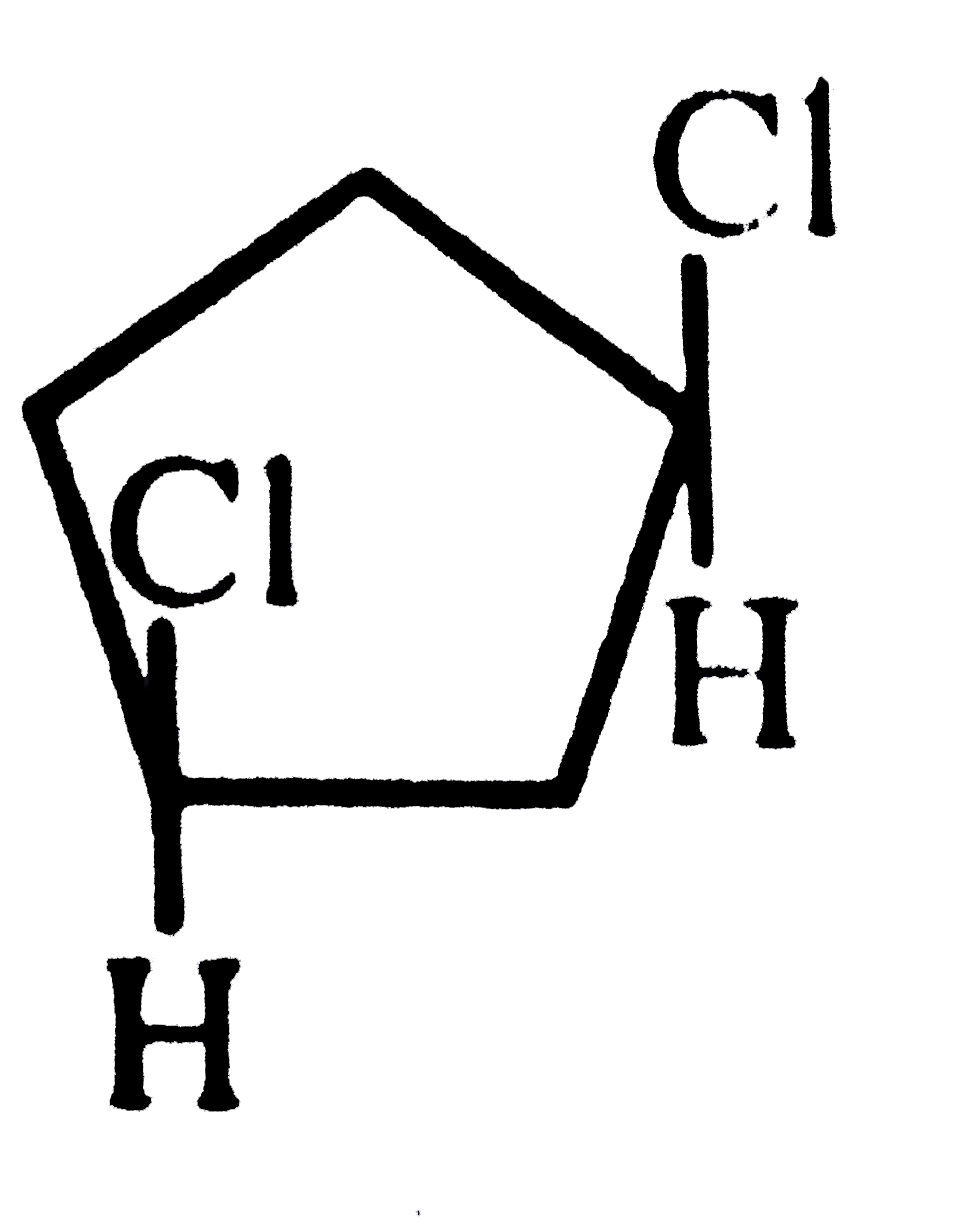
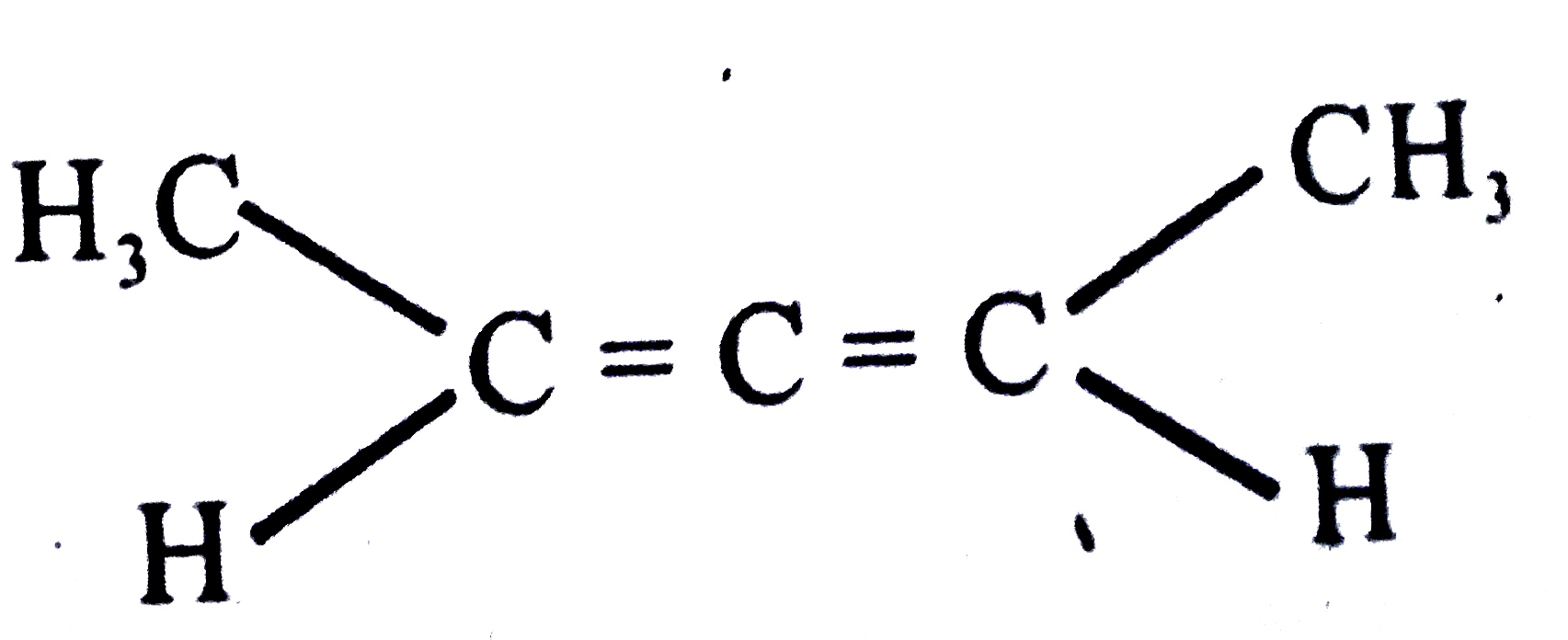A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPER 3-CHEMISTRY
- Which statement is true
Text Solution
|
- Which is favourable for homolysis :-
Text Solution
|
- Which of the following has plane of symmetry:-
Text Solution
|
- Which is optically active :-
Text Solution
|
- Given molecules are:-
Text Solution
|
- Which of the following is correct
Text Solution
|
- PQ(2) dissociates as PQ2 (g)hArrPQ(g)+Q(g) The initial pressure ...
Text Solution
|
- P hArr Q, K1 1/3RhArr 1/3Q,K2 2R hArr 2S, K3 P hArr S, K4 =? ...
Text Solution
|
- In the reaction , A(s) +B(g) + "heat" hArr 2C(s) + 2D(g) at equili...
Text Solution
|
- For which of the following gaseous reactions, the relationship log KC/...
Text Solution
|
- At which temperature, P C K K KP/KC value will be 1/4 for dissociatio...
Text Solution
|
- Equilibrium constants for (a) N2 + O2 hArr 2NO and (b) NO + 1/2O2 hArr...
Text Solution
|
- At t^@C temperature, the observed vapour density of A is 17.5 for the ...
Text Solution
|
- For the reaction A((s)) + 2B((g)) hArr 3C((g)) . At constant pressur...
Text Solution
|
- Which of following does not show common ion effect ?
Text Solution
|
- For the reaction 3A(g)+B(g) hArr 2C(g) at a given temperature , Kc=9.0...
Text Solution
|
- When A2 and B2 are allowed to react, the equilibrium constant of the r...
Text Solution
|
- The dissociation constant fo acetic acid at a given temperature is 1.6...
Text Solution
|
- 6.0 g weak acid HA (mol.mass=60 g/mol.) is dissolved in water and form...
Text Solution
|
- PbOhArrPb+1/2O2, K=2xx10^(-1) ZnOhArr +1/2 O2 K=2xx10^2 CuOhArr +...
Text Solution
|