Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-HALOALKANES AND HALOARENES-MULTIPLE CHOICE QUESTIONS (MCQS)
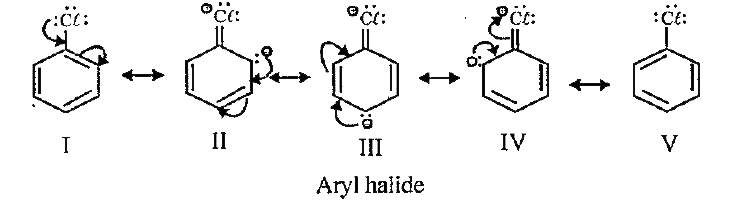
- With the help of resonance show that aryl halides are lesser reactive ...
Text Solution
|
- Ethyl alcohol gives ethyl chloride with the help of
Text Solution
|
- CH3 -CH =CH2 +HI to X where X is
Text Solution
|
- The reaction CH3 -CH =CH2 +HBr to CH3 - underset(Br) underse...
Text Solution
|
- SN^1 reaction of alkylhalides leads to
Text Solution
|
- Most reactive metal is:
Text Solution
|
- The reactivity order of halides for dehydrohalogenation is
Text Solution
|
- Mg reacts with RBr best in
Text Solution
|
- Both methane and ethane can be prepared in single step by the use of
Text Solution
|
- Alkyl halides react with Mg in dry ether to form
Text Solution
|
- Which of the following metal can be used for carrying out Wurtz-Fittig...
Text Solution
|
- Ethyl alcohol gives ethyl chloride with the help of
Text Solution
|
- CH3 -CH =CH2 +HI to X where X is
Text Solution
|
- The reaction CH3 -CH =CH2 +HBr to CH3 - underset(Br) underse...
Text Solution
|
- SN^1 reaction of alkylhalides leads to
Text Solution
|
- Most reactive halide towards SNI reaction is
Text Solution
|
- The reactivity order of halides for dehydrohalogenation is
Text Solution
|
- Mg reacts with RBr best in
Text Solution
|
- Both methane and ethane can be prepared in single step by the use of
Text Solution
|
- Alkyl halides react with Mg in dry ether to form
Text Solution
|
- Which of the following metal can be used for carrying out Wurtz-Fittig...
Text Solution
|