Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SWAN PUBLICATION-ATOMS AND MOLECULES-IMPORTANT Q/A
- How many molecules are present in 9g of water ?
Text Solution
|
- How many molecules are present in 17g of ammonia
Text Solution
|
- Calculate the number of moles in 17 g of H(2)O(2).
Text Solution
|
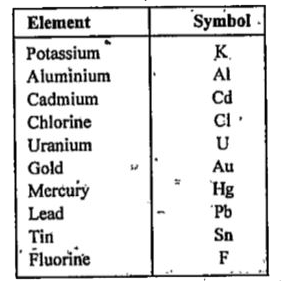
- Give symbols of the following elements: Potassium, aluminium, cadmiu...
Text Solution
|
- Calculate the molecular mass of the following CO(2)
Text Solution
|
- Calculate the molecular mass of the following (using atomic mass). N...
Text Solution
|
- Calculate the molecular mass of the following (using atomic mass). C...
Text Solution
|
- Calculate the molecular mass of the following (using atomic mass). H...
Text Solution
|
- Calculate the molecular mass of the following (using atomic mass). S...
Text Solution
|
- Calculate the molecular mass of the following (using atomic mass). H...
Text Solution
|
- Calculate the formula mass of the compounds whose formulae are given b...
Text Solution
|
- Calculate the formula mass of the compounds whose formulae are given b...
Text Solution
|
- Calculate the formula mass of the compounds whose formulae are given b...
Text Solution
|
- Calculate the formula mass of the compounds whose formulae are given b...
Text Solution
|
- Sodium carbonate (Na(2)CO(3).10H(2)O) is an important industrial chemi...
Text Solution
|
- What is the mass of : 1 mole of nitrogen atoms ?
Text Solution
|
- What is the mass of : 4 mole of Al atoms
Text Solution
|
- What is the mass of : 1.50 mol of Na^(+) ions
Text Solution
|
- What is the mass of : 10 moles of sodium sulphite (Na2SO3)?
Text Solution
|
- A sample of ammonia (NH(3)) weighs 2.00g. What mass of sulphur dioxide...
Text Solution
|