Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.5 Uses Of Aldehydes And Ketones)|3 VideosORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.6 Nomenclature And Structure of Carboxylic Group)|8 VideosORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.2 Preparation of Aldehydes And Ketones )|10 VideosHALOALKANES AND HALOARENES
BETTER CHOICE PUBLICATION|Exercise QUESTIONS|158 VideosPOLYMERS
BETTER CHOICE PUBLICATION|Exercise QUESTIONS |42 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II-Question Bank (12. 3 Physical Properties )
- How will you convert : Acetaldehyde to isopropyl alcohols
Text Solution
|
- How will you convert Benzene into acetophenone.
Text Solution
|
- How will you convert benzene to benzophenone ?
Text Solution
|
- How will you convert : CH(3)CN^(-) (Methyl cyanide) to CH(3)COCH(3)...
Text Solution
|
- How will you convert : Ethanol to propan -2-ol
Text Solution
|
- How will you convert : Formaldehyde to methyl alcohol
Text Solution
|
- How will you convert : Ethanol to propan -2-ol
Text Solution
|
- Give simple chemical test to distinguish between the following pairs o...
Text Solution
|
- Give simple chemical tests to distinguish between the following pairs ...
Text Solution
|
- How will you distinguish: Benzaldehyde and acetophenone
Text Solution
|
- How will you distinguish: Benzaldehyde and acetophenone
Text Solution
|
- Give simple chemical tests to distinguish between the following pairs ...
Text Solution
|
- Write a chemical test to distinguish between Acetic acid and Acetone.
Text Solution
|
- How will youdistinguish between benzaldehyde andacetaldehyde?
Text Solution
|
- What type of aldehyde and ketones undergo Cannizzaro's reaction ?
Text Solution
|
- Why is aldehydes group in benzaldehyde meta directing in nature ?
Text Solution
|
- Write chemical equation to show Popoff's rule.
Text Solution
|
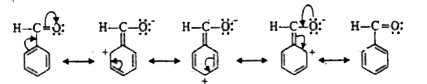
- Explain, why benzaldehyde is less reactive than aliphatic aldehydes?
Text Solution
|
- Aromatic aldehydes and ketones are less reactive than correspondingAli...
Text Solution
|
- Write short note on Haloform reaction.
Text Solution
|