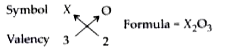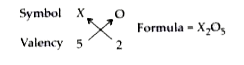Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise NCERT SECTION |25 VideosATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (MULTIPLE CHOICE QUESTIONS LEVEL-1) |50 VideosATOMS AND MOLECULES
MTG IIT JEE FOUNDATION|Exercise OLYMPIAD/HOTS CORNER |20 VideosCARBON AND ITS COMPOUNDS
MTG IIT JEE FOUNDATION|Exercise Exercise (Integer/Numerical Value Type)|5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-ATOMS AND MOLECULES -SOLVED EXAMPLES
- A piece of copper weighs 0.635 g. How many atoms of copper does it con...
Text Solution
|
- Calculate the mass of a single atom of sulphur and a single molecule o...
Text Solution
|
- What mass of silver nitrate will react with 5.85 g of sodium chloride ...
Text Solution
|
- Calculate the molecular masses of the following: (i) C(12)H(22)O(11)...
Text Solution
|
- An element X shows a variable valency of 3 and 5. What are the formula...
Text Solution
|
- Calculate formula unit mass of Na(2)CO(3).10H(2)O
Text Solution
|
- 10^(22) atoms of an element X are found to have a mass of 930 mg. Calc...
Text Solution
|
- Calculate the number of molecules present in 1 litre of water assuming...
Text Solution
|
- Calculate the mass percentage of composition of sulphur in calcium sul...
Text Solution
|
- Which amongst the following has more number of atoms? (i) 11.5 g of ...
Text Solution
|
- Calculate the number of atoms of each type that are present in 3.42 g ...
Text Solution
|
- Calculate mass of sodium which contains same number of atoms as are pr...
Text Solution
|
- Calculate the number of moles in the following: (i) 28 g of He (ii) ...
Text Solution
|
- Calculate the weight of carbon monoxide having the same number of oxyg...
Text Solution
|
- A flask P contains 0.5 mole of oxygen gas. Another flask Q contains 0....
Text Solution
|