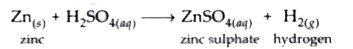Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
MTG IIT JEE FOUNDATION|Exercise EXERCISE (Multiple Choice Questions )|50 VideosCHEMICAL REACTIONS AND EQUATIONS
MTG IIT JEE FOUNDATION|Exercise EXERCISE ( Match the Following )|5 VideosCHEMICAL REACTIONS AND EQUATIONS
MTG IIT JEE FOUNDATION|Exercise EXERCISE ( Integer/Numerical Value Type )|8 VideosCARBON AND ITS COMPOUNDS
MTG IIT JEE FOUNDATION|Exercise Exercise (Integer/Numerical Value Type)|5 VideosFOOTSTEPS TOWARDS (JEE MAIN)
MTG IIT JEE FOUNDATION|Exercise SECTION B ( Numerical Value Type Questions)|10 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-CHEMICAL REACTIONS AND EQUATIONS-Solved Examples
- Mention the type of chemical reaction that takes place when : a magn...
Text Solution
|
- Mention the type of chemical reaction that takes place when : limest...
Text Solution
|
- Mention the type of chemical reaction that takes place when : electr...
Text Solution
|
- Give an example where corrosion is an advantage rather than a disadvan...
Text Solution
|
- Represent each of the following word equations with a balanced chemica...
Text Solution
|
- Represent each of the following word equations with a balanced chemica...
Text Solution
|
- What happens when zinc granules are treated with dilute solutio of H(2...
Text Solution
|
- Name the type of chemical reaction represented by the following equati...
Text Solution
|
- Name the type of chemical reaction represented by the following equati...
Text Solution
|
- Name the type of chemical reaction represented by the following equati...
Text Solution
|
- Why is respiration considered an exothermic reaction? Explain.
Text Solution
|
- Write chemical name and the formula of the brown gas produced during t...
Text Solution
|
- Why do chips manufactures flush bags of chips with gas such as nitroge...
Text Solution
|
- Write balanced chemical equations for the following chemical reactions...
Text Solution
|
- Write balanced chemical equations for the following chemical reactions...
Text Solution
|
- Write balanced chemical equations for the following chemical reactions...
Text Solution
|
- State the type of chemical reactions, represented by the following equ...
Text Solution
|
- State the type of chemical reactions, represented by the following equ...
Text Solution
|
- What is a reduction reaction? Identify the substances that are oxid...
Text Solution
|
- What is a reduction reaction? Identify the substances that are oxid...
Text Solution
|