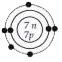
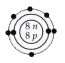
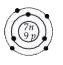
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
FOOTSTEPS TOWARDS(CBSE BOARD)
MTG IIT JEE FOUNDATION|Exercise SECTION-B|11 VideosFOOTSTEPS TOWARDS(CBSE BOARD)
MTG IIT JEE FOUNDATION|Exercise SECTION-C|15 VideosFOOTSTEPS TOWARDS (NEET)
MTG IIT JEE FOUNDATION|Exercise QUESTIONS|44 VideosIS MATTER AROUND US PURE ?
MTG IIT JEE FOUNDATION|Exercise OLYMPIAD/ HOTS CORNER|20 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-FOOTSTEPS TOWARDS(CBSE BOARD)-SECTION-D
- The maximum number of the electrons which are permitted to be assigned...
Text Solution
|
- Why did Rutherford select a gold foil in his alpha-ray scattering expe...
Text Solution
|
- Which of the two will be chemically more reactive? Element X with atom...
Text Solution
|
- Name the gas which produces anode rays consisting of protons in the di...
Text Solution
|
- The nucleus of a gaseous element is represented as ""(y)^(2y)X. This g...
Text Solution
|
- Atomic number does not change during a chemical reaction. Give reasons...
Text Solution
|
- (i) If an atom contains one electron and one proton, will it carry any...
Text Solution
|
- Given that natural sample of iron has isotopes ""(26)^(54) Fe, ""(26)^...
Text Solution
|
- if you are given a mixture of hydrogen and carbon dioxide, how would y...
Text Solution
|
- How would you separate: (i) benzene ( b.pt. 80^(@)C ) from toluene o...
Text Solution
|
- Write the names of the following compounds : (i) Al(2) ( SO(4))(3) ...
Text Solution
|
- A silver ornament of mass 'm' gram is polished with gold equivalent to...
Text Solution
|
- A sample of ethane ( C(2) H(6)) gas has the same mass as 1.5 xx 10^(2...
Text Solution
|
- Arrange the following in order of their increasing masses in grams. ...
Text Solution
|
- Which will weigh more 10^(23) molecules of oxygen or 10^(23) molecules...
Text Solution
|