A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
FOOTSTEPS TOWARDS (JEE MAIN)
MTG IIT JEE FOUNDATION|Exercise SECTION B ( Numerical Value Type Questions)|10 VideosCHEMICAL REACTIONS AND EQUATIONS
MTG IIT JEE FOUNDATION|Exercise EXERCISE ( Integer/Numerical Value Type )|8 VideosFOOTSTEPS TOWARDS (NEET)
MTG IIT JEE FOUNDATION|Exercise QUESTIONS|44 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-FOOTSTEPS TOWARDS (JEE MAIN)-SECTION B ( Numerical Value Type Questions)
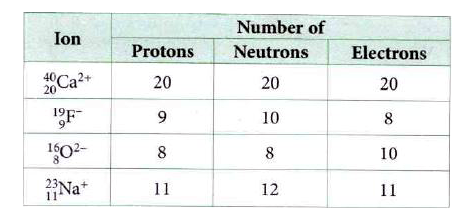
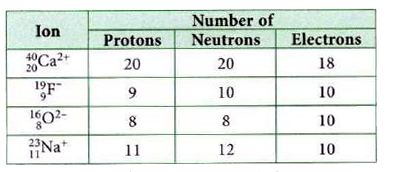
- The table given shows the number of protons, neutrons and electrons in...
Text Solution
|
- The number of sodium ions in 53g of Na(2)CO(3) is x xx 10^(23). The va...
Text Solution
|
- Calculate the mass of water ( in g ) required to make 250g of 25% solu...
Text Solution
|
- Ammonia gas diffuses x times faster than hydrogen chloride gas. The va...
Text Solution
|
- Number of protons , neutrons and electrons in four species A,B,X and Y...
Text Solution
|
- The formula of chloride of a metal M is MCl(3), then the number of oxy...
Text Solution
|
- A sample of water was heated from 50^(@)C to 328K. The rise in tempera...
Text Solution
|
- In the C-12 and C-14 isotopes, the ratio of neutrons is x: y , then x+...
Text Solution
|
- A compound contians 3 xx 10^(24) oxygen atoms. Number of moles of oxyg...
Text Solution
|
- The solubility of common salt in water at 293K is 36g. The mass ( in g...
Text Solution
|
- Mass of 270g block of iron displaces a volume of 30 mL of a liquid. Th...
Text Solution
|