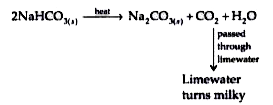Text Solution
Verified by Experts
Topper's Solved these Questions
ACIDS , BASES AND SALTS
MTG IIT JEE FOUNDATION|Exercise NCERT Section|43 VideosACIDS , BASES AND SALTS
MTG IIT JEE FOUNDATION|Exercise Exercise (Multiple Choice Questions) LEVEL -1|30 VideosACIDS , BASES AND SALTS
MTG IIT JEE FOUNDATION|Exercise Olympiad /HOTS Corner |20 VideosATOMIC STRUCTURE
MTG IIT JEE FOUNDATION|Exercise Exercise (Integer/Numerical Value Type)|5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-ACIDS , BASES AND SALTS -SOLVED EXAMPLES
- Give general equation for the reactions of acids with metal car...
Text Solution
|
- Give general equation for the reaction of acids with metal oxides
Text Solution
|
- How does the flow of acid rain water into a river make the surviva...
Text Solution
|
- Write the chemical formula of washing soda .What happens when crysta...
Text Solution
|
- A person found that the cake prepared by him is hard and small in size...
Text Solution
|
- Name the substance obtained by action of chlorine on dry slaked lime...
Text Solution
|
- A white powdered solid when added to water produces hissing sound ....
Text Solution
|
- Tooth enamel is one of the hardest substances in our body .How do...
Text Solution
|
- With the help of labelled diagrams, describe an activity to show that ...
Text Solution
|
- Write the chemical formula for bleaching powder .How is bleaching pow...
Text Solution
|
- How can you test that metal oxides are basic while non - metal oxide...
Text Solution
|
- How would you distinguish between baking powder and washing soda by he...
Text Solution
|
- Answer the following : (a) Why is Plaster of Paris written as CaSO(4...
Text Solution
|
- Answer the following : (a) Why is Plaster of Paris written as CaSO(4...
Text Solution
|
- When electricity is passed through an aqueous solution of sodium chl...
Text Solution
|
- Write the balanced equation in molecular form illustrating the compl...
Text Solution
|
- What happens when Bleaching powder reacts with dilute sulphuric ...
Text Solution
|
- Slaked lime reacts with chlorine to form
Text Solution
|
- Sodium hydrogencarbonate is heated .
Text Solution
|
- Gypsum is heated .
Text Solution
|