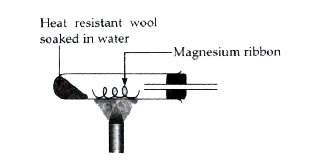
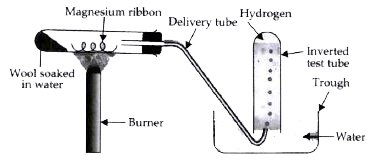
Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON METALS
MTG IIT JEE FOUNDATION|Exercise Exercise (Integer/Numerical Value Type )|5 VideosMETALS AND NON METALS
MTG IIT JEE FOUNDATION|Exercise Olympiad/HOTS Corner|20 VideosMETALS AND NON METALS
MTG IIT JEE FOUNDATION|Exercise Exercise (Subjective Problems) Short Answer Type |15 VideosFOOTSTEPS TOWARDS CBSE BOARD
MTG IIT JEE FOUNDATION|Exercise SECTION - C|39 VideosMOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE) |5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-METALS AND NON METALS -Exercise (Subjective Problems) Long Answer Type
- Explain the factors which promote corrosion.
Text Solution
|
- How does formation of alloys improve the properties of metals ?
Text Solution
|
- Why is galvanisation considered better than tinning?
Text Solution
|
- What is alloying of gold? Why is it done? What is meant by 24 carat go...
Text Solution
|
- (a) Write the steps involved in the extraction of pure metals in the...
Text Solution
|
- Define an alloy and an amalgam. State the main constituents of the fol...
Text Solution
|
- Explain extraction of magnesium by electrolytic reduction method.
Text Solution
|
- Give reasons for the following : (a) Silicon counts among metalloids...
Text Solution
|
- Magnesium reacts with steam to form magnesium oxide and a gas. ...
Text Solution
|
- What happens when (a) Calcium reacts with water (b) Iron reacts w...
Text Solution
|