A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
METALS AND NON METALS
MTG IIT JEE FOUNDATION|Exercise Exercise (Integer/Numerical Value Type )|5 VideosFOOTSTEPS TOWARDS CBSE BOARD
MTG IIT JEE FOUNDATION|Exercise SECTION - C|39 VideosMOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE) |5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-METALS AND NON METALS -Olympiad/HOTS Corner
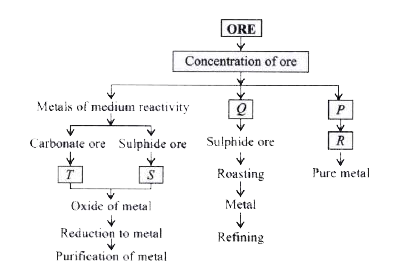
- Metals are extracted from their ores by different methods depending on...
Text Solution
|
- Metals are extracted from their ores by different methods depending on...
Text Solution
|
- Which one of the following oxides gives pink colour with phenolphthale...
Text Solution
|
- Solder is an alloy of
Text Solution
|
- The given apparatus shows the reaction of steam with heated solid 'X'....
Text Solution
|
- Observe the given figure carefully. For which pair of metals woul...
Text Solution
|
- Which gas is released when a metal reacts with an acid ?
Text Solution
|
- When in the blue solution of Copper sulphate zinc strip is dipped, aft...
Text Solution
|
- Extraction of highly electropositive metal is done by
Text Solution
|
- Some properties of substances P, Q, R and S are given in the table : ...
Text Solution
|
- Match column-I with column-II and select the correct option using the ...
Text Solution
|
- Ms. Neelam, a class 10 teacher took Cu, Al, Pb and Zn strips respecti...
Text Solution
|
- Which reagent is able to dissolve gold and platinum ?
Text Solution
|
- Amalgam is
Text Solution
|
- A few chemical properties of three metals X, Y and Z are summarised in...
Text Solution
|
- Mitaali, a class 10 student wanted to study the speed of reactions of ...
Text Solution
|
- A metal rod M was dipped in a coloured solution Y. After some time it ...
Text Solution
|
- is a non-metal but is lustrous.
Text Solution
|
- Main objective of smelting of ore is
Text Solution
|
- A metal X is placed below Al and above Pb, in activity series. The ext...
Text Solution
|