Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise Solved Examples|37 VideosPERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise NCERT Section|35 VideosMOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE) |5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-PERIODIC CLASSIFICATION OF ELEMENTS-Olympiad/HOTS Corner
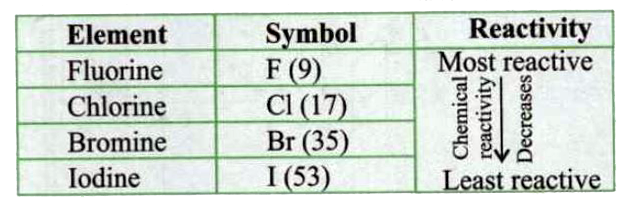
- While going down in a group of non-metals the chemical reactivity decr...
Text Solution
|
- The positions of four elements K, L, M and N in the periodic table are...
Text Solution
|
- Which of the following orders of atomic radii is correctly represented...
Text Solution
|
- Which of the given elements A,B,C,D and E with atomic number 2,3,7,10 ...
Text Solution
|
- Elements M forms an ion, M^(2+) and element X forms an ion, X^(2-). Th...
Text Solution
|
- Periodicity in the properties of elements in modern periodic table is ...
Text Solution
|
- X, Y and Z are the three elements, each one belongs to any one of the ...
Text Solution
|
- Which of the following statements is not a correct statement about the...
Text Solution
|
- The given part of the modern periodic table shows positions of element...
Text Solution
|
- The elements A, B, C and D have atomic numbers 4,12, 17 and 19 respect...
Text Solution
|
- Which of the following metals is not placed in eighth group of Mendele...
Text Solution
|
- The atoms having the bigger size among each of the following pairs are...
Text Solution
|
- Which of the following elements would lose an electron easily ?
Text Solution
|
- Example of Dobereiner's triad is
Text Solution
|
- The given table shows a part of the periodic table P, Q, R, T and...
Text Solution
|
- An element X belongs to group 2 and period 3 of the periodic table. Th...
Text Solution
|