Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise Exercise (Subjective Problems) (Long Answer Type)|13 VideosPERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise Exercise (Integer/Numerical Value Type)|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise Exercise (Subjective Problems Very Short Answer Type)|26 VideosMOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE) |5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-PERIODIC CLASSIFICATION OF ELEMENTS-Exercise (Subjective Problems) (Short Answer Type)
- Chlorine is an element in period 3 of the Periodic Table. Bromine is f...
Text Solution
|
- Chlorine is an element in period 3 of the Periodic Table. Bromine is f...
Text Solution
|
- Why atomic size increases on going down the group? Arrange the followi...
Text Solution
|
- Potassium bromine and krypton are elements in period 4 of the Periodic...
Text Solution
|
- Potassium bromine and krypton are elements in period 4 of the Periodic...
Text Solution
|
- Potassium bromine and krypton are elements in period 4 of the Periodic...
Text Solution
|
- An element X has a total of 31 nucleons, out of which 16 are neutrons....
Text Solution
|
- An element X has a total of 31 nucleons, out of which 16 are neutrons....
Text Solution
|
- An element X has a total of 31 nucleons, out of which 16 are neutrons....
Text Solution
|
- What physical and chemical properties of elements were used by Mendele...
Text Solution
|
- Table given below shows a part of the periodic table Using this t...
Text Solution
|
- Table given below shows a part of the periodic table Using this t...
Text Solution
|
- Table given below shows a part of the periodic table Using this t...
Text Solution
|
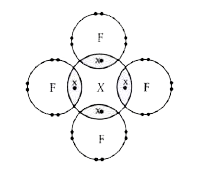
- The given diagram shows the electron arrangement in a compound formed ...
Text Solution
|
- The given diagram shows the electron arrangement in a compound formed ...
Text Solution
|
- The given diagram shows the electron arrangement in a compound formed ...
Text Solution
|
- Study the variation in the atomic radii of first group elements given ...
Text Solution
|
- Study the variation in the atomic radii of first group elements given ...
Text Solution
|
- Atomic number is considered to be a more appropriate parameter than at...
Text Solution
|
- Atomic number is considered to be a more appropriate parameter than at...
Text Solution
|