Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise Exercise (Integer/Numerical Value Type)|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise Olympiad/HOTS Corner|15 VideosPERIODIC CLASSIFICATION OF ELEMENTS
MTG IIT JEE FOUNDATION|Exercise Exercise (Subjective Problems) (Short Answer Type)|23 VideosMOLE CONCEPT, STOICHIOMETRY AND BEHAVIOUR OF GASES
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE) |5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-PERIODIC CLASSIFICATION OF ELEMENTS-Exercise (Subjective Problems) (Long Answer Type)
- An atom is electrically neutral but still it has a tendency to form an...
Text Solution
|
- Why do we classify elements?
Text Solution
|
- What were the two criteria used by Mendeleev in creating his periodic ...
Text Solution
|
- Why did Mendeleev leave some vacant places in his periodic table ?
Text Solution
|
- In Mendeleev's periodic table, why were noble gases like helium, neon ...
Text Solution
|
- Would you place the two isotopes of chlorine, Cl-35 and Cl-37 in diffe...
Text Solution
|
- Why is argon bigger than chlorine inspite of the fact that atomic radi...
Text Solution
|
- Taking an example of element of 3rd period discuss the trend of reacti...
Text Solution
|
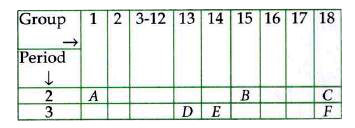
- Study the following table in which positions of six elements A, B, C, ...
Text Solution
|
- Study the following table in which positions of six elements A, B, C, ...
Text Solution
|
- Study the following table in which positions of six elements A, B, C, ...
Text Solution
|
- Study the following table in which positions of six elements A, B, C, ...
Text Solution
|
- Study the following table in which positions of six elements A, B, C, ...
Text Solution
|