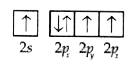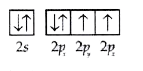A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
MTG IIT JEE FOUNDATION|Exercise Exercise (Match the following) |5 VideosATOMIC STRUCTURE
MTG IIT JEE FOUNDATION|Exercise Exercise (Assertion & Reason Type)|10 VideosATOMIC STRUCTURE
MTG IIT JEE FOUNDATION|Exercise Solved Examples |11 VideosACIDS , BASES AND SALTS
MTG IIT JEE FOUNDATION|Exercise Olympiad /HOTS Corner |20 VideosCARBON AND ITS COMPOUNDS
MTG IIT JEE FOUNDATION|Exercise Olympaid/HOTS Corner|25 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-ATOMIC STRUCTURE -Exercise (Multiple Choice Question)
- The outermost electronic configuration of the most electronegative ele...
Text Solution
|
- No two electrons in an atom of an element have
Text Solution
|
- the orbital diagram in which aufbau principal is violated is :
Text Solution
|
- If uncertainty in possition of electron is zero, then the uncertainty...
Text Solution
|
- de-Broglie equation tells about
Text Solution
|
- When an electron jumps from L to K shell -
Text Solution
|
- Which of the following orbitals does not exist ?
Text Solution
|
- Which of the following is not possible ?
Text Solution
|
- For 4s-orbital, m has the value
Text Solution
|
- Presence of three unpaired electrons in phosphorus atom can be explain...
Text Solution
|
- The maximum number of electrons in a subshell is given by the expressi...
Text Solution
|
- Which of the following relates to photon both as wave motion and as a...
Text Solution
|
- The number of nodes in 5d orbital is
Text Solution
|
- For which of the following species, Bohr theory is not applicable ?
Text Solution
|
- If quantum number for last electron is n=3,l=0,m=0, then atomic number...
Text Solution
|
- Which of the following transitions have minimum wavelength
Text Solution
|
- Planck's constant has the dimension (unit) of
Text Solution
|
- When the frequency of light incident an a metallic plate is doubled , ...
Text Solution
|
- The wavelength (in nanometer) associated with a proton moving at 1.0xx...
Text Solution
|
- A 0.66 kg ball is moving with a speed of 100 m/s. The associated wavel...
Text Solution
|