Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING
MTG IIT JEE FOUNDATION|Exercise Solved Examples |10 VideosCHEMICAL BONDING
MTG IIT JEE FOUNDATION|Exercise Exercise (Multiple Choice Questions)|50 VideosCARBON AND ITS COMPOUNDS
MTG IIT JEE FOUNDATION|Exercise Olympaid/HOTS Corner|25 VideosCHEMICAL EQUILIBRIUM
MTG IIT JEE FOUNDATION|Exercise EXERCISE (INTEGER/NUMERICAL VALUE TYPE)|5 Videos
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-CHEMICAL BONDING -Exercise (Integer/Numerical Value Type )
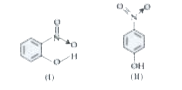
- Which of the above two compounds will show higher melting point?
Text Solution
|
- The number of electrons present in 'L' shell of neon is
Text Solution
|
- The total number of covalent bonds present in methane is
Text Solution
|
- The number of electrons present in outermost shell of halogen is
Text Solution
|
- Number of lone electron pairs around central atom in BrF5 is
Text Solution
|
- The number of electrons sulphur require to acquire octet structure is
Text Solution
|