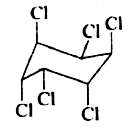
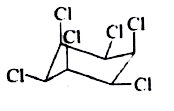
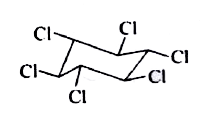
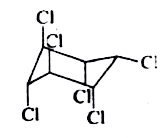
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM AND REACTION MECHANISM
BRILLIANT PUBLICATION|Exercise LEVEL-II |40 VideosISOMERISM AND REACTION MECHANISM
BRILLIANT PUBLICATION|Exercise LEVEL - III |8 VideosHYDROGEN
BRILLIANT PUBLICATION|Exercise LEVEL -III ( Linked Comprehension Type)|8 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART I (NOMENCLATURE)
BRILLIANT PUBLICATION|Exercise LEVEL-III|45 Videos
Similar Questions
Explore conceptually related problems