A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM AND REACTION MECHANISM
BRILLIANT PUBLICATION|Exercise LEVEL - III |8 VideosISOMERISM AND REACTION MECHANISM
BRILLIANT PUBLICATION|Exercise LEVEL - III (Multiple choice answer type)|15 VideosISOMERISM AND REACTION MECHANISM
BRILLIANT PUBLICATION|Exercise LEVEL - III (Linked Comprehension Type) (Paragraph III )|3 VideosHYDROGEN
BRILLIANT PUBLICATION|Exercise LEVEL -III ( Linked Comprehension Type)|8 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART I (NOMENCLATURE)
BRILLIANT PUBLICATION|Exercise LEVEL-III|45 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-ISOMERISM AND REACTION MECHANISM-LEVEL-II
- Which will undergo fastest S(N)2 substitution reaction when treated wi...
Text Solution
|
- Under identical conditions, S(N)1 reaction will occur most efficient w...
Text Solution
|
- Consider the reaction, RCHO+NH(2)NH(2)rarrRCH=N-NH(2). What type of re...
Text Solution
|
- An incorrect statement with respect to S(N)1 and S(N)2 mechanism for a...
Text Solution
|
- In which of the following molecules, the resonance effect is not prese...
Text Solution
|
- For 1-methoxy-1, 3-butadiene, which of the following resonating struct...
Text Solution
|
- Which of the following statements regarding resonance is not correct?
Text Solution
|
- Amongst the given species, the best leaving group in a nucleophilic su...
Text Solution
|
- The mechanism of the reaction between tert-butyl alcohol and hydroxide...
Text Solution
|
- In which of the following pairs A is more stable than B?
Text Solution
|
- Which of the following carbocations will not rearrange?
Text Solution
|
- Which one of the following substitutents at para-position is most effe...
Text Solution
|
- The order of decreasing ease of abstraction of hydrogen atoms in the f...
Text Solution
|
- Which of the following reactions involves a nucleophile? (I) CH(3)CO...
Text Solution
|
- In the following carbocation, H//CH(3) that is most likely to migrate...
Text Solution
|
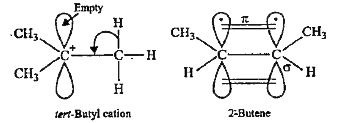
- The hyperconjugative stabilities of tert-butyl cation and 2-butene, re...
Text Solution
|
- If the carbocation rearranges to gain stability, it will rearrange to
Text Solution
|
- p-chlorophenol is a stronger acid than phenol because
Text Solution
|
- The major product in the reaction is:
Text Solution
|
- Consider the following compounds: Hyperconjugation occurs in
Text Solution
|