A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-II|102 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion-Reason Type)|18 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-III|52 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-III ( Linked Comprehension Type ))|12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL THERMODYNAMICS -LEVEL-I
- The Haber's process for production of ammonia involves the equilibrium...
Text Solution
|
- For a particular reaction, Delta H ^(@) =- 38.3 kJ and Delta S ^(@) =-...
Text Solution
|
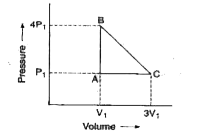
- An ideal gas is taken around the cycle ABCA as: The work done in ...
Text Solution
|
- Assuming that water vapour is an ideal gas, the internal energy (Delta...
Text Solution
|
- A piston filled with 0.04 mol of an ideal gas expands reversibly from ...
Text Solution
|
- 'Which of the following statement is not correct?
Text Solution
|
- What is the molar heat capacity of ethyl alcohol, C (2) H (5) OH, in u...
Text Solution
|
- If at 298 K the bond enèrgies of C-H, C-C, C=C and H - H are respectiv...
Text Solution
|
- In an irreversible process taking place at constant T and P and in whi...
Text Solution
|
- Oxygen gas weighing 64 g is expanded from 1 atm to 0.25 atm at 30^(@)C...
Text Solution
|
- For the process H (2) O (l) ⇔H (2) O (g) at T = 100 ^(@)C and 1 atmosp...
Text Solution
|
- The enthalpy of a reaction at 273 K is-4.58 kJ. What will be the entha...
Text Solution
|
- The gas absorbs 150 J heat and is simultaneously compressed by a const...
Text Solution
|
- The densities of graphite and diamond at298K are 2.25gcm and 3.31 gcm^...
Text Solution
|
- Consider 100 mL of a liquid contained in an isolated container at a pr...
Text Solution
|
- Consider the given reaction 2 CO + O (2) to 2 CO (2) , Delta H =-560 ...
Text Solution
|
- Nitrogen dioxide, NO(2) an air pollutant, dissolves in rain-water to ...
Text Solution
|
- The standard Gibbs energy change for a reaction isDelta G ^(@) =- 41.8...
Text Solution
|
- The enthalpy change for a reaction does not depend upon
Text Solution
|
- For a spontaneous reaction, the DeltaG, equilibrium constant (K) and E...
Text Solution
|