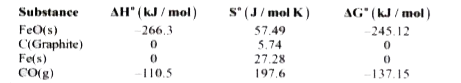A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion-Reason Type)|18 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-III|52 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-I|92 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-III ( Linked Comprehension Type ))|12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL THERMODYNAMICS -LEVEL-II
- The standard enthalpy of formation of NH(3) is -46 kJ mol^(-1). If the...
Text Solution
|
- Fe (2 ) O (3 (s)) + (3)/(2) C to (3)/(2) CO (2 (g)) + 2 Fe ((s)) , Del...
Text Solution
|
- Given the following data: Determine at what temperature the follo...
Text Solution
|
- Calculate the increase in internal energy of 1 kg of water at 100^(@)C...
Text Solution
|
- Oxidizing power of chlorine in aqueous solution can be determined by t...
Text Solution
|
- A steam boiler made up of steel weighs 900 kg. The boiler contains 400...
Text Solution
|
- If for the reaction at 300 K, 2 Mg ((s)) + O (2 (g)) to 2 Mg O ((s)) ,...
Text Solution
|
- The lattice energy of NaCl is +788 kJ mol^(-1). The enthalpies of hydr...
Text Solution
|
- Using the data given below, pick the correct statement about the react...
Text Solution
|
- which of the following statements is correct?
Text Solution
|
- H (2 (g)) + (1)/(2) O (2) to H (2) O ((l)) , Delta H (298 K) = - 68.32...
Text Solution
|
- Given that,NH (3 (g)) + 3 Cl (2 (g)) hArr NCl (3 (g)) + 3 HCl ((g)) , ...
Text Solution
|
- One mole of an ideal gas at 300 K is heated at constant volume (V(1) )...
Text Solution
|
- 1 mol of an ideal gas undergoes reversible isothermal expansion from a...
Text Solution
|
- The heat evolved on combustion of 1g starch (C (6) H (10) O (5)) (n) i...
Text Solution
|
- Given the following thermo chemical equations: 2NO ((g)) + O (2 (g))...
Text Solution
|
- Diborane is a potential rocket fuel which undergoes combustion accordi...
Text Solution
|
- Calculate the equilibrium constant for a reaction at 400 K given that ...
Text Solution
|
- The net work done through a series of changes reported in figure for a...
Text Solution
|
- If Delta G = Delta H - T Delta S and Delta G = Delta H + T [ (d (Delta...
Text Solution
|