A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion-Reason Type)|18 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-III|52 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-I|92 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-III ( Linked Comprehension Type ))|12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL THERMODYNAMICS -LEVEL-II
- The heat evolved on combustion of 1g starch (C (6) H (10) O (5)) (n) i...
Text Solution
|
- Given the following thermo chemical equations: 2NO ((g)) + O (2 (g))...
Text Solution
|
- Diborane is a potential rocket fuel which undergoes combustion accordi...
Text Solution
|
- Calculate the equilibrium constant for a reaction at 400 K given that ...
Text Solution
|
- The net work done through a series of changes reported in figure for a...
Text Solution
|
- If Delta G = Delta H - T Delta S and Delta G = Delta H + T [ (d (Delta...
Text Solution
|
- The standard heat of combustion of Al is–837.8 kJ mol^(-1) at 25^(@)C....
Text Solution
|
- H (2 (g)) + (1)/(2) O (2 (g)) to H (2) O ((l)) , BE (H-H) = x(1), BE (...
Text Solution
|
- A piston filled with 0.04 mol of an ideal gas expands reversibly from ...
Text Solution
|
- If for an ideal gas, the ratio of pressure and volume is constant and ...
Text Solution
|
- Two cylinders Aand B fitted with pistons contain equal amounts of an i...
Text Solution
|
- 4 kg of ice at-20^(@)C is mixed with 10 kg of water at 20^(@)C in an i...
Text Solution
|
- If Delta (f) H ^(@) (CO,g) =- 110.5 kJ mol ^(-1) and Delta (f) H ^(@) ...
Text Solution
|
- The enthalpy of combustion of H(2)(g) at 298 K to give H(2)O(g) is -24...
Text Solution
|
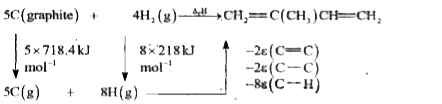
- If bond enthalpies of (C- H) = 413 kJ mol ^(-1) , (C -C) = 347. 7 kJ m...
Text Solution
|
- If Delta (f) G ^(@) (H(2)O, l) = - 237 . 19 kJ mol ^(-1) and Delta (f...
Text Solution
|
- 36 mL of pure water takes 100 sec to evaporate from a vessel and heate...
Text Solution
|
- Consider the reaction at 300 K, C (6) H (6) (l) + (15)/(2) O (2) (g) t...
Text Solution
|
- A rigid and insulated tank of 3 m^3 volume is divided into two compart...
Text Solution
|
- The enthalpy of neutralization of a weak monoprotic acid (HA) in 1 M s...
Text Solution
|