A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion-Reason Type)|18 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-III ( Linked Comprehension Type ))|12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL THERMODYNAMICS -LEVEL-III
- 100 mole of an ideal gas at 8.21 atm is heated to show a linear graph ...
Text Solution
|
- match the following
Text Solution
|
Text Solution
|
- match the following
Text Solution
|
- Match the transformations in Column I with appropriate options in Colu...
Text Solution
|
- Match the thermodynamic processes given under Column I with expression...
Text Solution
|
- Statement 1 : The solubility of most salts in water increases with ris...
Text Solution
|
- Statement 1 : An exothermic process which is non spontaneous at high t...
Text Solution
|
- Statement 1 : For an isothermal reversible process Q=-Wi.e. work done ...
Text Solution
|
- Statement 1 : When hydrogen gas at high pressure and room temperature ...
Text Solution
|
- Statement 1 : If both Delta H ^(@) and Delta S ^(@) are positive, then...
Text Solution
|
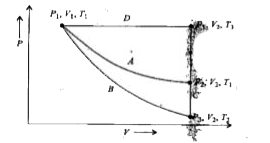
- For an ideal gas, an illustration of three different paths A, (B +C) a...
Text Solution
|
- For an ideal gas, an illustration of three different paths A, (B +C) a...
Text Solution
|
- For an ideal gas, an illustration of three different paths A, (B +C) a...
Text Solution
|
- Free energy, G=- TS, is a state funtion that indicates whether a react...
Text Solution
|
- Which of the following is trur for the reaction ? H (2) O (1) hArr H...
Text Solution
|
- One mol of ice is converted to liquid at 273 K : H (2) O (s) and H (2)...
Text Solution
|
- In which of the following cases Delta H and DeltaU are not equal to ea...
Text Solution
|
- The latent heat of vaporization of liquid at 500 K and 1 atmospheric p...
Text Solution
|
- One mole of a gas is allowed to expand freely in vacuum at 300 K. The ...
Text Solution
|