A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III|19 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level - III (Numerical type)|8 VideosCHEMICAL AND IONIC EQUILIBRIUM
BRILLIANT PUBLICATION|Exercise Level -I|54 VideosCHEMICAL BONDING
BRILLIANT PUBLICATION|Exercise LEVEL-III ( Linked Comprehension Type )|12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL AND IONIC EQUILIBRIUM-Level -II
- At constant temperature, the equilibrium constant (K(P)) for the decom...
Text Solution
|
- An acid-base indicator (PK(a) =4.5271) has the acid form red and basi...
Text Solution
|
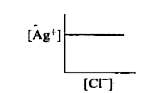
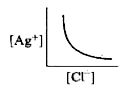
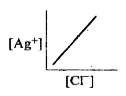
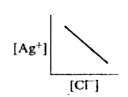
- In a saturated solution of AgCl, addition of NaCl is made drop by drop...
Text Solution
|
- 0.1 M solution of H(3)A being a weak triprotic acid having K (a (1)) ,...
Text Solution
|
- When equal volumes of the following solutions are mixed, precipitation...
Text Solution
|
- 2.5 mL of (2M)/(5) weak monoacidic base (K(b) = 1xx10^(-12) at 25^(@)...
Text Solution
|
- The ionization constant of NH(4)^(+) in water is 5.6 xx 10^(-10) at 25...
Text Solution
|
- The pH of blood stream is maintained by a proper balance of H(2) CO(3)...
Text Solution
|
- The solubility of Pb(OH)(2) in water is 6.7 xx10^(-6) M Calculate sol...
Text Solution
|
- The K (sp ) of Ca (OH) (2) is 4.42 xx 10 ^(-4) at 25 ^(@)C. A 500 mL ...
Text Solution
|
- An aqueous solution of a metal bromide MBr(2) (0.05 M) is saturated wi...
Text Solution
|
- What volume must 1L of 0. 5 M CH (3) COOHsolution should be diluted wi...
Text Solution
|
- The pH curve the titration of weak acid with a strong base is given be...
Text Solution
|
- H(2)S is bubbled into 0.2 M NaCN solution which is 0.02 M in each [Cd ...
Text Solution
|
- For the equilibrium system 2HX (g) hArr H (2) (g) +X (2) (g) the equi...
Text Solution
|
- The %yield of ammonia as a function of time in the reaction N (2) (g) ...
Text Solution
|
- For the reaction N (2) O (4) (g) hArr 2NO (2) (g), the value of K (p) ...
Text Solution
|
- 100 mL of a buffer solution contains 0.1 M each of weak acid HA and sa...
Text Solution
|
- The solubility products of MA,MB,MC, and MD are 1.8 xx10^(-10), 4 xx10...
Text Solution
|
- CaCO (3) and BaCO (3) have solubility product values 1xx10^(-8) and 5...
Text Solution
|