A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-II|48 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Single Correct Answer Type)|7 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise (Leve-2)|24 VideosHALOALKANES AND HALOARENES
BRILLIANT PUBLICATION|Exercise Level-II|37 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type) |11 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-NITROGEN COMPOUNDS-LEVEL-I
- The final product formed when ethyl amine is treated with NaNO2 and HC...
Text Solution
|
- Conversion of benzene diazonium chloride to chlorobenzene is an exampl...
Text Solution
|
- Arrange the following compounds in decreasing order of their basic cha...
Text Solution
|
- What is the end product in the following sequence of reactions? Ace...
Text Solution
|
- Which of the following cannot produce hydrogen when treated with metal...
Text Solution
|
- Consider the synthesis below. What is reagent Z?
Text Solution
|
- The reduction of which of the following compounds would yield secondar...
Text Solution
|
- Lowest boiling point will be of the compound
Text Solution
|
- Which of the following can be detected by carbylamine reaction-
Text Solution
|
- Which of the following is produced by reducing RCN in sodium and alcoh...
Text Solution
|
- When a solution of aliphatic amine is treated with HNO2 the effervesce...
Text Solution
|
- The compound which on reaction with aqueous nitrous acid at a low temp...
Text Solution
|
- Which of the following can not give Hoffmann's bromamide reaction:
Text Solution
|
- Which of the following is the weakest Bronsted base?
Text Solution
|
- In the nitration of benzene using a mixture of conc. H2 SO4 and conc. ...
Text Solution
|
- The most reactive amine towards dilute hydrochloric acid is .............
Text Solution
|
- Best method for preparing aliphatic primary amines from alkyl halides ...
Text Solution
|
- The electrolytic reduction of nitrobenzene in strongly acidic medium p...
Text Solution
|
- m-Bromoaniline can be prepared by
Text Solution
|
- The following reaction is known by the name
Text Solution
|
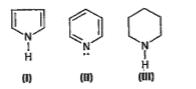
 , Pyrrole is an aromatic compound but both the lone pair electrons contribute to the `pi`-electron system. The lone pair, thus, takes part in resonance and is delocalized over the ring. Hence, it is less available for a proton and the basic character is the least.
, Pyrrole is an aromatic compound but both the lone pair electrons contribute to the `pi`-electron system. The lone pair, thus, takes part in resonance and is delocalized over the ring. Hence, it is less available for a proton and the basic character is the least.  Pyridine is an aromatic system with six-`pi`-electrons, so the ring is planar and lone pair is held `sp^2` hybridized orbital of nitrogen. The increased s character of this orbital as compared to the `sp^3` orbital in piperidine means that the lone pair electrons are held closer to the nitrogen and hence less available for protonation. Hence the basicity is less than that of piperidine.
Pyridine is an aromatic system with six-`pi`-electrons, so the ring is planar and lone pair is held `sp^2` hybridized orbital of nitrogen. The increased s character of this orbital as compared to the `sp^3` orbital in piperidine means that the lone pair electrons are held closer to the nitrogen and hence less available for protonation. Hence the basicity is less than that of piperidine. 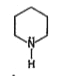 , In piperidine, nitrogen is `sp^3` hybridized, so the lone pair is held in `sp^3` orbital. It is more basic than pyridine where the lone pair resides in `sp^2` orbital. Hence, the order is `III gt II gt I`
, In piperidine, nitrogen is `sp^3` hybridized, so the lone pair is held in `sp^3` orbital. It is more basic than pyridine where the lone pair resides in `sp^2` orbital. Hence, the order is `III gt II gt I`