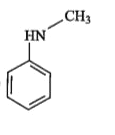
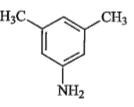
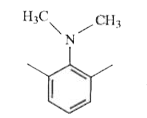
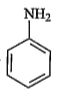A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Single Correct Answer Type)|7 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Multiple Choice Answer Type)|12 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-I|50 VideosHALOALKANES AND HALOARENES
BRILLIANT PUBLICATION|Exercise Level-II|37 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type) |11 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-NITROGEN COMPOUNDS-LEVEL-II
- Rank the following compounds in terms of their relative reactivity in ...
Text Solution
|
- The aromatic heterocyclic base pyridine is sulphonated by heating with...
Text Solution
|
- In which of the following compounds is there the least delocalization ...
Text Solution
|
- Give the reagents that will best accomplish the following transformati...
Text Solution
|
- Which of the following statements is/are true about amines?
Text Solution
|
- Arrange the following structure of quaternary salt in the decreasing o...
Text Solution
|
- Which of the following statements are correct? 1.In Sandmeyer reacti...
Text Solution
|
- Indicate which nitrogen compound amongst the following would undergo H...
Text Solution
|
- Reagents capable of converting cyclohexanone to N-ethyl cyclohexylamin...
Text Solution
|
- Which has the highest value of pkb ?
Text Solution
|
- C4 H(11) N on reaction with HNO2 forms tertiary alcohol. Thus, C4 H(11...
Text Solution
|
Text Solution
|
- Hofmann's elimination product of
Text Solution
|
- Basic nature of the following is in order
Text Solution
|
- The major products from the following sequence of reactions are (CH...
Text Solution
|
- What is the product of the following reaction?
Text Solution
|
- The reaction of chloroform with alcoholic KOH and p-toluidine forms
Text Solution
|
- In the chemical reaction the compounds A and B, respectively are
Text Solution
|
- Which of the following is the correct statement about coupling reactio...
Text Solution
|
- Identify the end product Z in the following sequence. C6 H5 NH2 ...
Text Solution
|