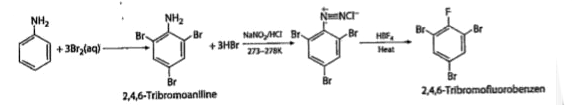
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Single Correct Answer Type)|7 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Multiple Choice Answer Type)|12 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-I|50 VideosHALOALKANES AND HALOARENES
BRILLIANT PUBLICATION|Exercise Level-II|37 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type) |11 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-NITROGEN COMPOUNDS-LEVEL-II
- Identify the end product Z in the following sequence. C6 H5 NH2 ...
Text Solution
|
- Arrange the following amines in order of increasing basicity (least to...
Text Solution
|
- Aniline is treated with bromine water and the resulting product is tr...
Text Solution
|
- In the above sequence, II is
Text Solution
|
- Determine the end product of the following reactions C2 H5 NH2 over...
Text Solution
|
- Benzylamine may be alkylated as shown in the following equation: C6...
Text Solution
|
- Which of the following reagents would not be a good choice for reducin...
Text Solution
|
- Amongst the given set of reactants, the most appropriate for preparing...
Text Solution
|
- Which of the following methods of preparation of amines will not give ...
Text Solution
|
- The product 'Y' in the following reaction sequence is
Text Solution
|
- Which one of the following nitro-compounds does not react with nitrous...
Text Solution
|
- An organic compound A upon reacting with NH3 gives B. On heating, B gi...
Text Solution
|
- In a set of reactions, m-bromobenzoic acid gave a product D. Identify ...
Text Solution
|
- The correct statement regarding the basicity of arylamines is
Text Solution
|
- An organic compound 'A' on reduction gives compound 'B' which on react...
Text Solution
|
- Although amino group ls-o- and p- directing in aromatic electrophilic ...
Text Solution
|
- The correct sequence of reactions to be performed to convert benzene i...
Text Solution
|
- An amine reacts with benzene sulphonyl chloride to form a white precip...
Text Solution
|
- When propane is subjected to the treatment with fuming nitric acid at ...
Text Solution
|
- A nitrogenous substance (X) is treated with HNO, and the product so fo...
Text Solution
|