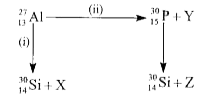A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-II|38 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Single Correct Answer Type ) |8 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type) |11 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type)|9 VideosP-BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise LEVEL-III|50 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-NUCLEAR CHEMISTRY -LEVEL-I
- A positron is emitted by ""(11)^(23) Na. The ratio of the atomic mass ...
Text Solution
|
- The total no. of alpha and beta- particles emitted in the nuclear reac...
Text Solution
|
- Bombardment of aluminium by alpha-particles leads to the artifical dis...
Text Solution
|
- The number of neutrons in the parent nucleus which gives ""(7)^(14)N o...
Text Solution
|
- The triad of nuclei that is isotonic is
Text Solution
|
- Which of the following nucleus is unstable ?
Text Solution
|
- The radioactive isotope tritum (""(3)^(1)H) has a half life 0f 12.3 ye...
Text Solution
|
- If 8.0 g of radioactive isotope has a half-life of 10 h, the half-life...
Text Solution
|
- Which one of the following particles is used to bombard ""(13)^(27)Al ...
Text Solution
|
- Which of the following nuclides are most likely to be neutron poor ?
Text Solution
|
- The half-life period of a radioactive element is 140 days. After 560 d...
Text Solution
|
- A nuclear reaction must be balanced in terms of
Text Solution
|
- If half-life of a substance is 5 years, then the total amount of subs...
Text Solution
|
- The element 'X' in the following reaction is : ""(90)^(234)Th to 7(2)^...
Text Solution
|
- A radioactive element gets spilled over the floor of a room, Its half-...
Text Solution
|
- You have 0.1 gram-atom of a radioactive isotope ""(Z)^(A) X (half-life...
Text Solution
|
- The observed massof ""(26)^(56)Fe is 55.9375 amu. Using the masses of...
Text Solution
|
- Emission of a beta -particle by an atom of an element results in the f...
Text Solution
|
- The 4n + 2 disintegration series is also known as
Text Solution
|
- The radioactive series to which ""(88)^(228) Ra belongs is
Text Solution
|