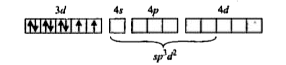
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL II|50 VideosCOORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL III (Single Correct Answer Type )|10 VideosCOORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION- REASON TYPE )|20 VideosCO-ORDINATION COMPOUNDS AND ORGANOMETALLICS
BRILLIANT PUBLICATION|Exercise Level-II (Assertion- Reason)|4 VideosD & F BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise Level -II|38 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-COORDINATION COMPOUNDS -LEVEL I
- The pair having the same magnetic moment is : (Atomic Nos : Cr=24, M...
Text Solution
|
- Hybridization, shape and magnetic moment of K3[Co(CO)3] is
Text Solution
|
- Which one of the following is an outer orbital complex and exhibits pa...
Text Solution
|
- Which one of the following is wrongly matched?
Text Solution
|
- Geometrical shape of the most stable complexes formed by the reaction ...
Text Solution
|
- The low spin complex of d^(6) metal ion in an octahedral field will ha...
Text Solution
|
- Which ofthe following complexions is expected to absorb visible light?
Text Solution
|
- [Cr(H(2)O)(6)]Cl(3) has a spin only magnetic moment of 3.83BM. The cor...
Text Solution
|
- Among the following complexes, the one which shows zero CFSE is (Ato...
Text Solution
|
- EAN of cobalt is 36 in [Co(NH3)2 O2 (en)Cl]. Knowing that atomic numbe...
Text Solution
|
- Fe2(CO)9 is diamagnetic. Which of the following is the correct reason?...
Text Solution
|
- Among the following metal carbonyls, the C–O bond order is lowest in
Text Solution
|
- Which of the following complexes is used as an anti-cancer agent?
Text Solution
|
- Sodium nitroprusside reacts with sulphide ion to give a purple colour ...
Text Solution
|
- Find the ligand having the highest denticity from the following option...
Text Solution
|
- Which of the following ligands is tridentate type?
Text Solution
|
- Which of the following complex has highest EAN value?
Text Solution
|
- Which of the following complexes contains a cationic ligand?
Text Solution
|
- In all the following complexes, the coordination number of iron is six...
Text Solution
|
- Consider the coordination compound, [Co(NH3)6]Cl3. In the formation of...
Text Solution
|