Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL III (Matching Column Type)|4 VideosCOORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL III (Statement Type)|5 VideosCOORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL III (Multiple Correct Answer Type)|12 VideosCO-ORDINATION COMPOUNDS AND ORGANOMETALLICS
BRILLIANT PUBLICATION|Exercise Level-II (Assertion- Reason)|4 VideosD & F BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise Level -II|38 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-COORDINATION COMPOUNDS -LEVEL III (Numerical Type)
- For the octahedral complexes of Fe^(3+) in SCN^(-) (thiocyanato-S) and...
Text Solution
|
- Among the complex ions, [Co(NH2CH2 CH2 NH2 )2 Cl2]^(+), [CrCl2(C2O4)...
Text Solution
|
- In the complex acetylbromidodicarbonylbis (triethyl phosphine) iron (I...
Text Solution
|
- The number of geometrical isomers possible for the complex [CoL2Cl2]^(...
Text Solution
|
- The possible number of stereoisomers for the formula [Ma2b3]^(n pm) is
Text Solution
|
- The EAN of M(CO)x is 36. If atomic number of metal is 24, then x is ……...
Text Solution
|
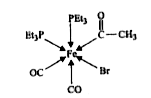
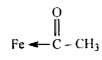 bond. Total number of Fe - C bonds = 3.
bond. Total number of Fe - C bonds = 3.