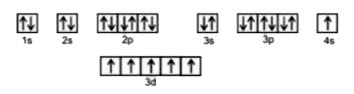
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-STRUCTURE OF ATOM TEST -1-MULTIPLE CHOICE QUESTIONS
- The electron energy in a hydrogen atom is given by En = (-2.18 xx 10^(...
Text Solution
|
- By calculating the wavelength for the emission transition, if it start...
Text Solution
|
- Calculate the minimum and the maximum number of electrons which may ha...
Text Solution
|
- the wavelength of light that must be absorbed to excite a hydrogen ele...
Text Solution
|
- The ionization energy of gaseous Na atoms is 495.5 kJ "mol"^(-1). The ...
Text Solution
|
- For a hypothetical hydrogen like atom, the potential energy of the sys...
Text Solution
|
- Which transition in the hydrogen atomic spectrum will have the same wa...
Text Solution
|
- The energy of second Bohr orbit of the hydrogen atom is -328 K kJ "mol...
Text Solution
|
- The work function (phi) of some metals is listed below. The number of ...
Text Solution
|
- Which of the following statements in relation to the hydrogen atom is ...
Text Solution
|
- An electron jumps from an outer orbit to an inner orbit with an energy...
Text Solution
|
- The shortest wavelength of H atom in Lyman series is x, then the longe...
Text Solution
|
- Calculate the angular momentum of an electron in the third orbit of a ...
Text Solution
|
- For a certain atom, there are energy levels A, B, C corresponds to ene...
Text Solution
|
- If the uncertainty in the position of a particle is equal to its de-Br...
Text Solution
|
- Rutherford's alpha particle dispersion experiment concludes
Text Solution
|
- If E1 ,E2 and E3 represent, respectively, the kinetic energies of an e...
Text Solution
|
- If the nitrogen atom had electronic configuration 1s^7 it would have e...
Text Solution
|
- According to the Bohr's Theory, which of the following transitions in ...
Text Solution
|
- The radiation with maximum frequency is
Text Solution
|