Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA TPC JEE MAIN TEST 47-CHEMISTRY (SUBJECTIVE NUMERICAL)
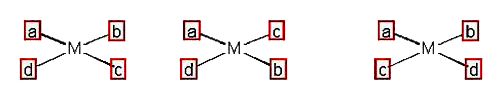
- Possible number of isomers of [Pt(Cl)(NO(2)) (NO(3))(SCN)]^(2-), give...
Text Solution
|
- Assuming the ideal geometries without any distortions, find the value...
Text Solution
|
- The total number of ores of aluminium present in the following mineral...
Text Solution
|
- In the crown like structure of a rhombic sulphur, the total number of ...
Text Solution
|
- How many moles of Grignard reagent will be consumed during the followi...
Text Solution
|
- A molecule of stachyose contains ....... carbon atoms.
Text Solution
|
- 3 – Methylbutan-2-ol +HI overset(Delta)to X Find the position of th...
Text Solution
|
- How many of the following will show isomerism? Butane, propane, he...
Text Solution
|
- XeF(4) and SbF(5) forms [XeF(x)]^(+) [SbF(y)]^(-). Then the possible ...
Text Solution
|