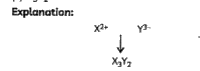A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
EDUCART PUBLICATION-SAMPLE PAPER 4-SECTION C
- Study the given table: X and Y combine to form a compound of form...
Text Solution
|
- Case 1: When an element composed of atoms that readily lose electrons ...
Text Solution
|
- Case 1: When an element composed of atoms that readily lose electrons ...
Text Solution
|
- Case 1: When an element composed of atoms that readily lose electrons ...
Text Solution
|
- Case 1: When an element composed of atoms that readily lose electrons ...
Text Solution
|