A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
EDUCART PUBLICATION-SAMPLE PAPER 4-SECTION B
- Select the correct equation (s) which represent double displacement r...
Text Solution
|
- Study the table below which shows different colour produced by univers...
Text Solution
|
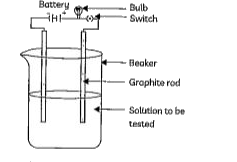
- An experiment was performed to test the electrical conductivity of som...
Text Solution
|
- Following metals are taken and each of them was tested for its reactio...
Text Solution
|
- What happens when dilute hydrochloric acid is added to iron filling:
Text Solution
|
- Assertion (A): When hydrogen and chlorine are placed in sunlight, hydr...
Text Solution
|
- Assertion (A): Most reactive metals reacts with dilute acids to libera...
Text Solution
|
- Study the table below and select the row that has the correct informat...
Text Solution
|
- A compound Z is manufactured by the action of a gas X, which is a prod...
Text Solution
|