Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 3-SECTION-B
- Derive Bragg's equation .
Text Solution
|
- What is catalysis ? How is catalysis classified ? Give two examples fo...
Text Solution
|
- Explain the following given examples. Colloid
Text Solution
|
- What is the definition of acid according to Bronsted?
Text Solution
|
- Define mole fraction.
Text Solution
|
- Calculate the molarity of a solution contaịning 10g of NaOH in 500 ml ...
Text Solution
|
- How are XeF(2) and XeF(4) prepared ? Give their structures.
Text Solution
|
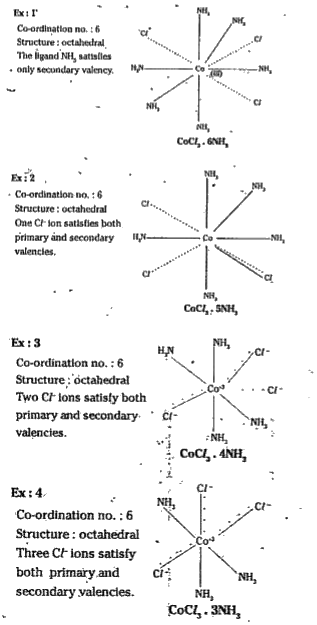
- Explain Werner's theory. Give the Werner's structures of CoCl3. 6NH3 ,...
Text Solution
|
- Give one example for corm.
Text Solution
|
- What are Hormones ? Give one example for steroid hormones and polypept...
Text Solution
|
- What are Hormones ? Give one example for each. i) Steroid Hormones ...
Text Solution
|
- Explain Wurtz - Fitting reaction
Text Solution
|
- Write equations of the following reactions: (i) Friedel-Crafts react...
Text Solution
|