Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 6-SECTION - C
- How is nitric acid manufactured by Ostwald's process ?
Text Solution
|
- How is ozone prepared from oxygen ? Explain its reaction i) C(2)H(4)...
Text Solution
|
- Give a detailed account of the Collision theory of reaction rates of b...
Text Solution
|
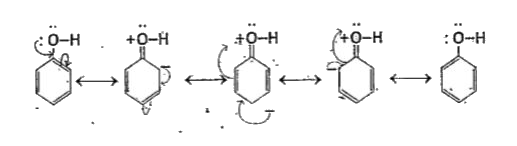
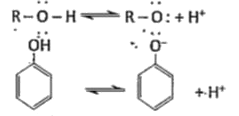
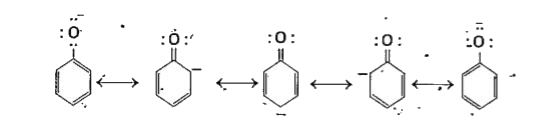
- Explain the acidic nature of phenols and compare with that of alcohols...
Text Solution
|
- With a suitable example write equations for the Kolbe's reaction.
Text Solution
|
- With a suitable example write equations for the Reimer-Tiemann reacti...
Text Solution
|