Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 7-SECTION-C
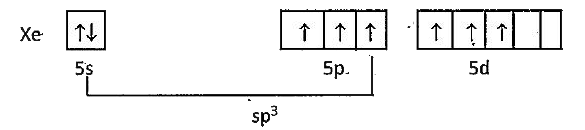
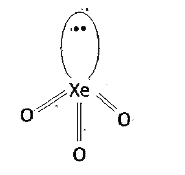
- Explain the structure of XeO(3).
Text Solution
|
- State and explain Kohlrausch's law of indendent migration of ions.
Text Solution
|
- What is "molecularity" of a reaction ? How is it different from the 'o...
Text Solution
|
- How is ozone prepared ? How does it react with the following ? PbS
Text Solution
|
- How is ozone prepared ? How does it react with the following ? KI
Text Solution
|
- How is ozone prepared ? How does it react with the following ? Hg
Text Solution
|
- How is ozone prepared ? How does it react with the following ? Ag
Text Solution
|
- How can you prepare Cl(2) from HCl and HCl from Cl(2) ? Write the reac...
Text Solution
|
- Write the equations involved in the following reactions: (i) Reimer ...
Text Solution
|
- Explain the following reactions. Williamson's Ether Synthesis Aldol ...
Text Solution
|
- Discuss aldol condensation.
Text Solution
|
- Describe the Decarboxylation .
Text Solution
|