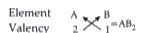Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise BOARD CORNER (Long Answer Type Questions) |7 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise NCERT EXEMPLAR (LONG ANSWER QUESTIONS)|36 VideosOLYMPIAD 2019 - 20
OSWAL PUBLICATION|Exercise CHEMISTRY QUESTIONS (Is Matter Around us Pure)|1 VideosSAMPLE PAPER 1
OSWAL PUBLICATION|Exercise QUESTION BANK|9 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -BOARD CORNER (Short Answer Type Questions)
- Based on the group valency of elements write the molecular formula of ...
Text Solution
|
- Based on the group valency of elements write the molecular formula of ...
Text Solution
|
- Based on the group valency of elements write the molecular formula of ...
Text Solution
|
- Write the names given to the vertical columns and horizontal rows in t...
Text Solution
|
- An element P (atomic number 20) reacts with an element Q (atomic numbe...
Text Solution
|
- Write the number of periods and groups in the Modern Periodic Table. H...
Text Solution
|
- Write the number of periods and groups in the Modern Periodic Table. H...
Text Solution
|
- Na, Mg and Al are the elements of the 3rd period of the Modern Periodi...
Text Solution
|
- Na, Mg and Al are the elements of the 3rd period of the Modern Periodi...
Text Solution
|
- Na, Mg and Al are the elements of the 3rd period of the Modern Periodi...
Text Solution
|
- What is periodicity in properties of elements with reference to the Mo...
Text Solution
|
- Write the electronic configuration of two elements X and Y whose atomi...
Text Solution
|