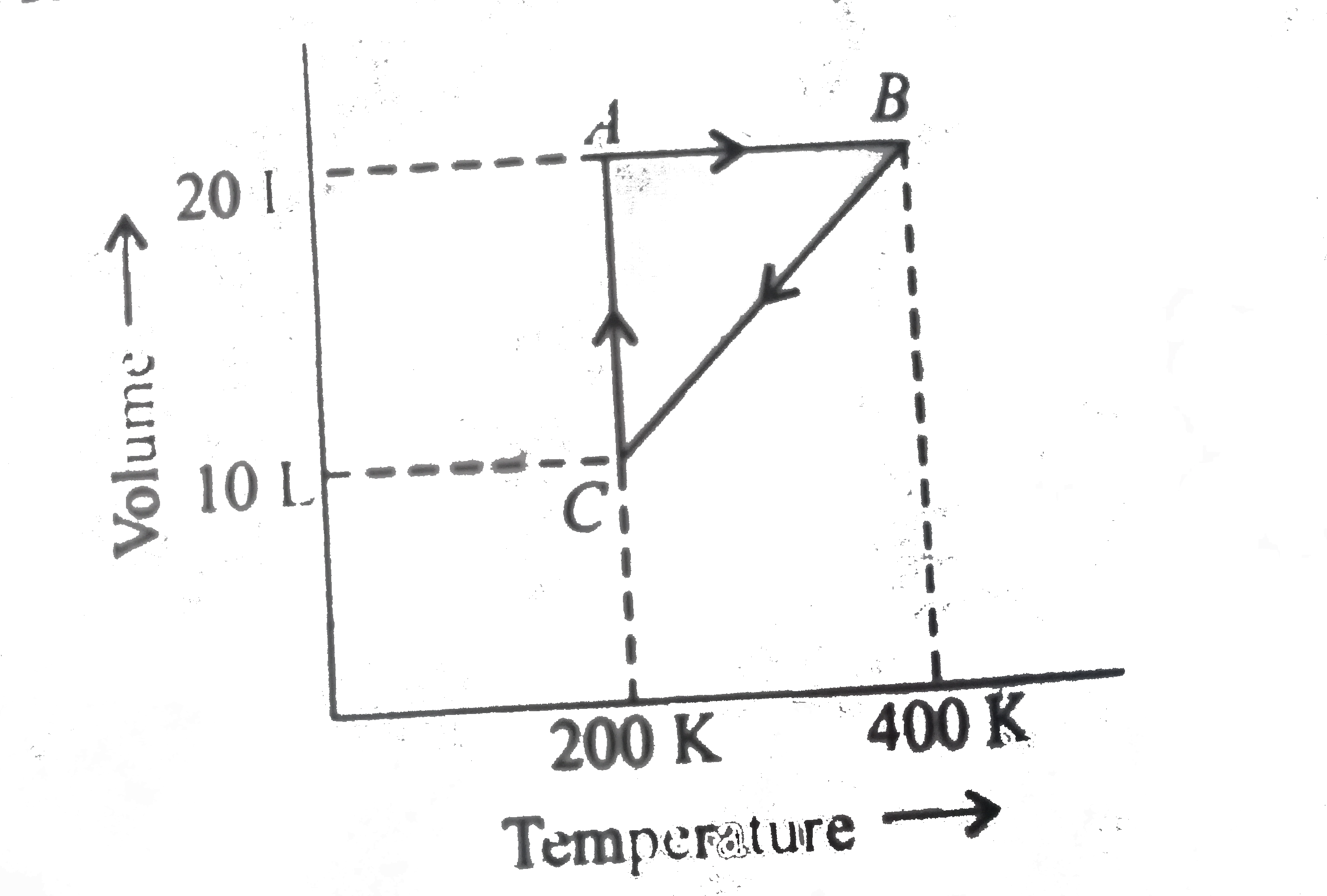
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROSTATICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - I) (LEVEL - II (ADVANCED ) MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)|1 VideosELECTROSTATICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - I) (LEVEL - II (ADANCED) INTEGER TYPE QUESTIONS)|3 VideosELECTROSTATICS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)|5 VideosELECTROMAGNETICS
AAKASH SERIES|Exercise ADDITIONAL EXERCISE|13 VideosELEMENTS OF VECTORS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTROSTATICS-LECTURE SHEET (EXERCISE - I) (LEVEL - II (ADVANCED ) STRAIGHT OBJECTIVE TYPE QUESTIONS)
- -10mu C, 40 mu C and q are the charges on three identical conductors ...
Text Solution
|
- Three charges-q(1),+q(2) and -q(3) are placed as shown in the figure. ...
Text Solution
|
- Equal charges Q are placed at the four corners A, B, C, D of a square ...
Text Solution
|
- The pressures at A and B in the atmosphere are, respectively,
Text Solution
|