Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-Set III
- Write the structure of 3-Bromo-2-methylprop-1-ene
Text Solution
|
- Write IUPAC name of the following compound (CH(3))(2) N - CH(2) CH(3...
Text Solution
|
- Write the reactions involved in the following reactions: (i) Clemmen...
Text Solution
|
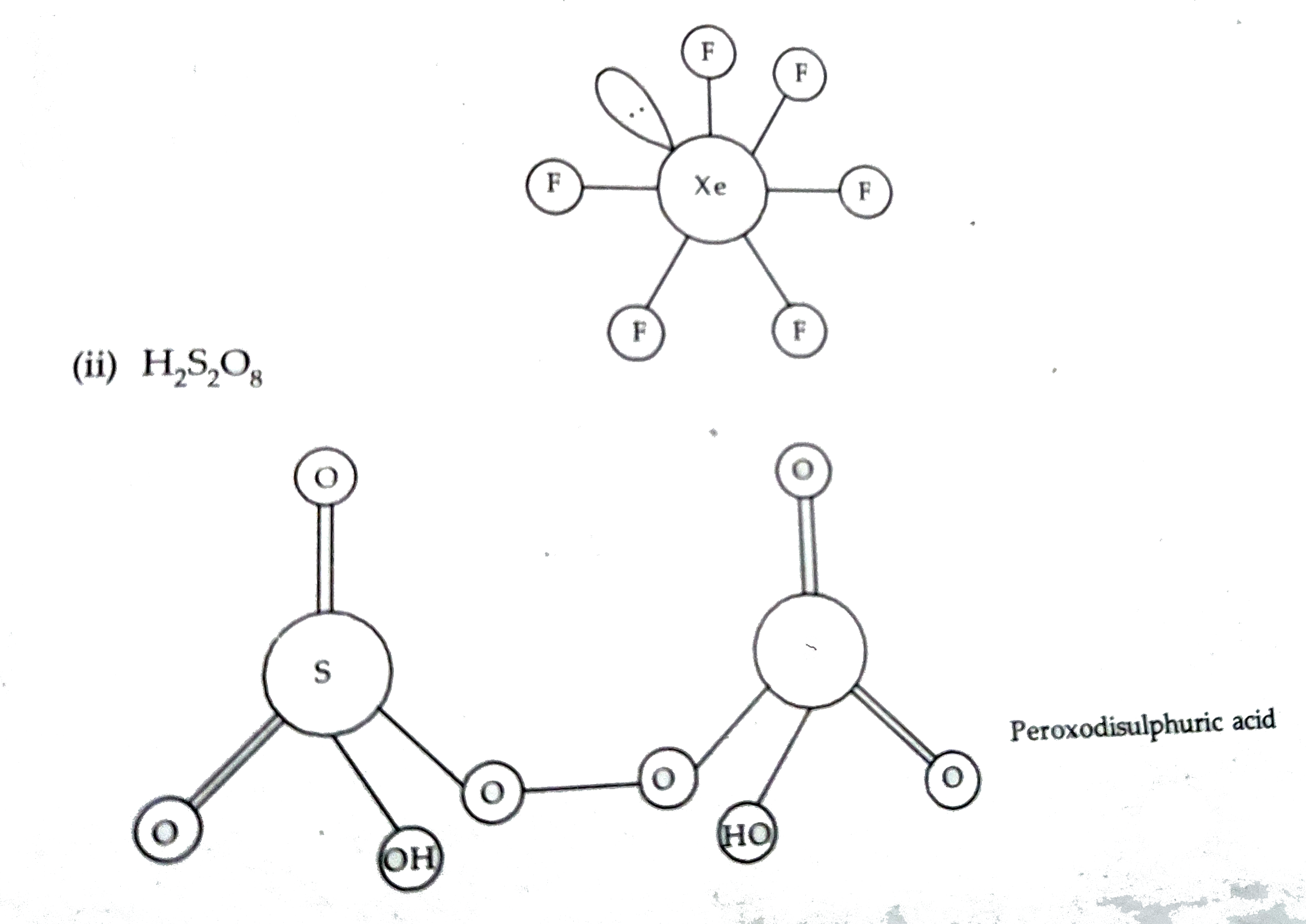
- Draw the structures of the following : (i) H(4)P(2)O(7) (ii) XeOF(...
Text Solution
|
- Define the following terms: (i) Abnormal molar mass (ii) Van't Hoff ...
Text Solution
|
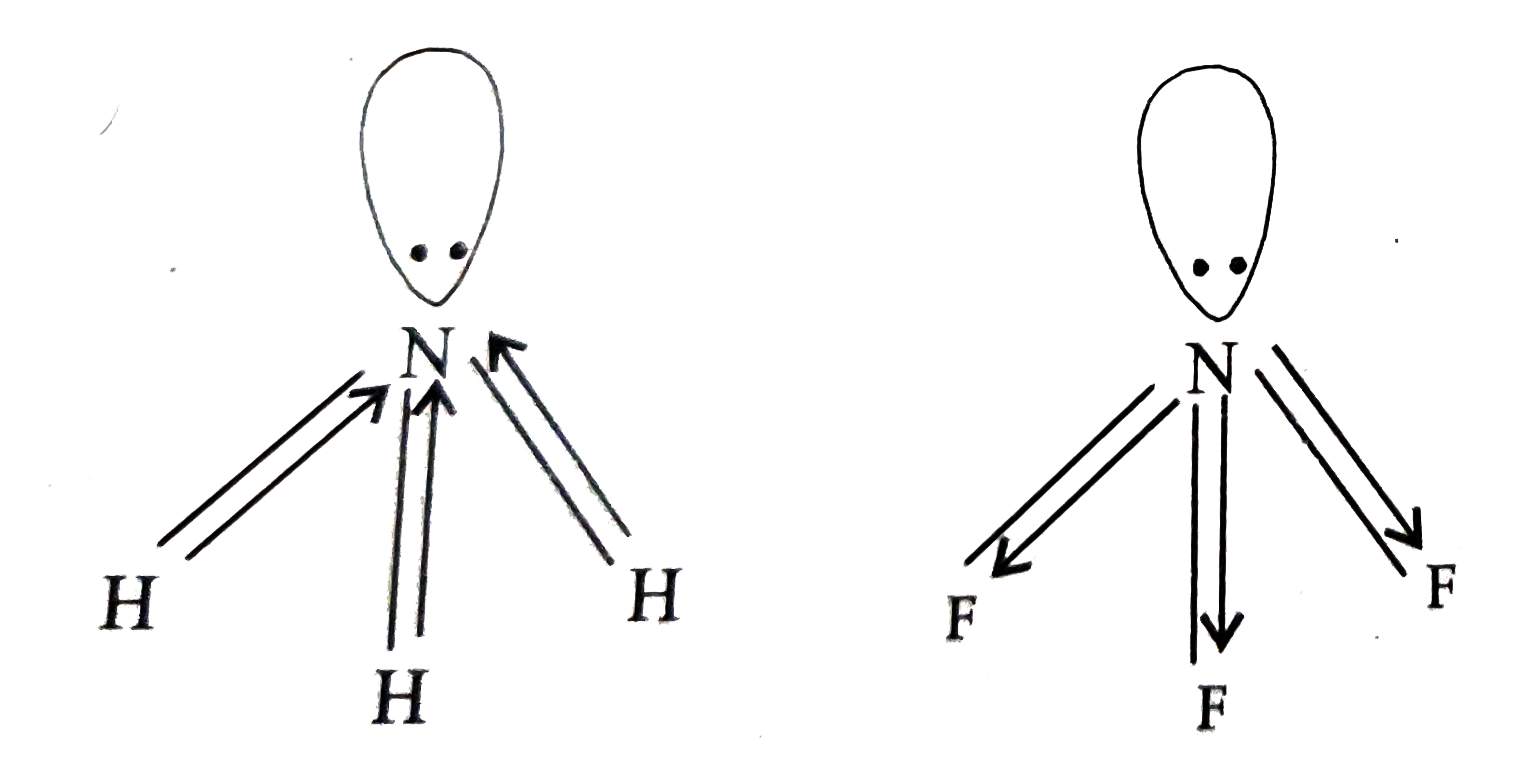
- Complete the following chemical equation (i) F(2) + 2Cl^(-) rarr (...
Text Solution
|
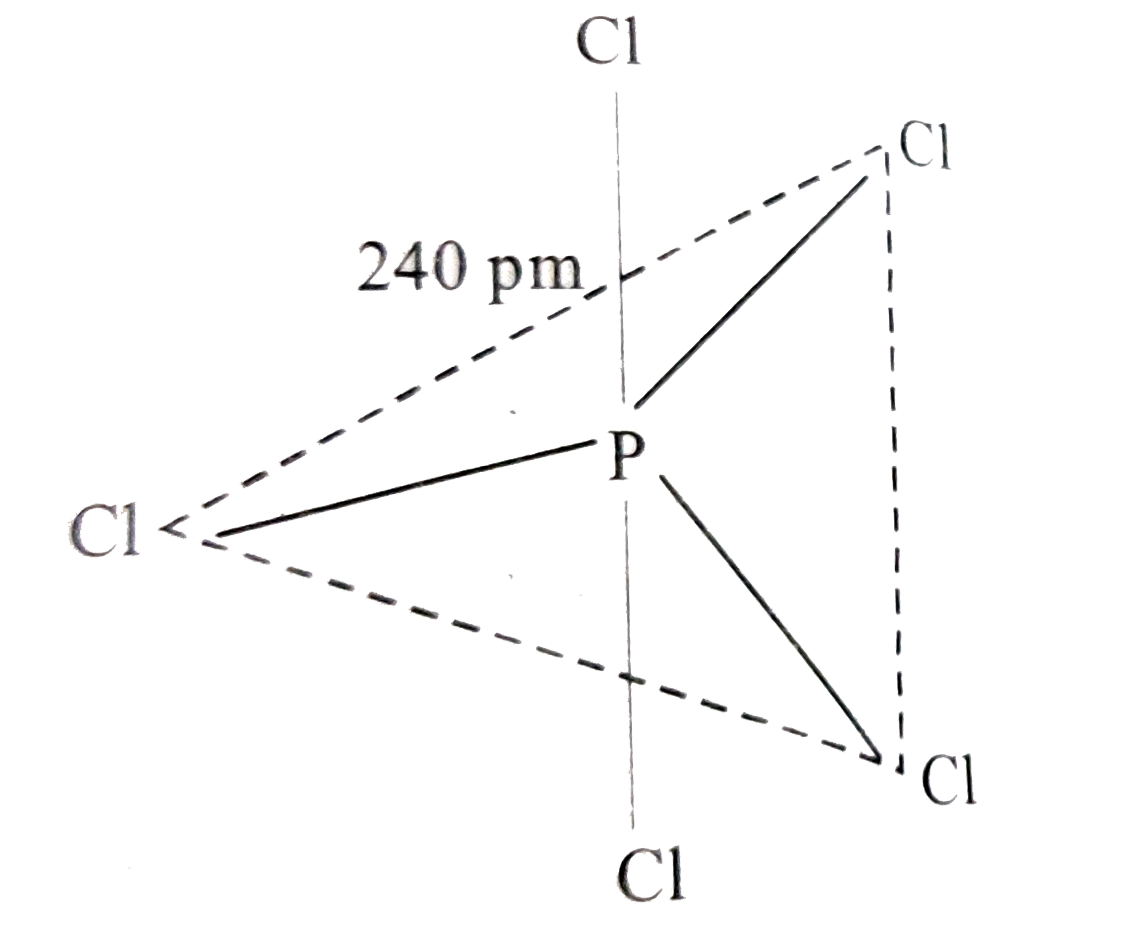
- What happens when (i) HCl is added to MnO(2)? (ii) PCl(5) is heated ...
Text Solution
|
- Define the following (i) Anionic detergents (ii) Limited spectrum ...
Text Solution
|
- Write the structure of the monomers used for getting the following pol...
Text Solution
|
- Write one difference between each of the following: (i) Multimolecul...
Text Solution
|
- (i) What type of isomerism is shown by complex [Co(en)(3)]Cl(3)? (ii...
Text Solution
|
- Of PH(3) and H(2)S which is more acidic and why ?
Text Solution
|
- Draw the structure of hex-1-en-3-ol compound.
Text Solution
|
- Explain the following terms giving one example for each : (i) Miscel...
Text Solution
|
- 15.0 g of an unknown molecular material was dissolved in 450 g of wate...
Text Solution
|
- Explain the following observations giving an appropriate reason for ea...
Text Solution
|
- Write the name, the structure and the magnetic behaviour of each one o...
Text Solution
|
- Explain the following terms giving one example of each type . (i) An...
Text Solution
|
- (a) Draw the molecular structure of following compounds : (i) XeF(6)...
Text Solution
|
- (a) Complete the following chemical equations . (i) XeF(4) + SbF(5) ...
Text Solution
|